Unless you've been in a long coma, you've undoubtedly heard of Ozempic.
Ozempic is Here... and Here to Stay for a While
Scientifically known as semaglutide, Ozempic is a drug that's taken the world by storm, chiefly because of its successful weight management results. Originally developed to manage type-2 diabetes, it's in a class of prescription drugs known as GLP-1 receptor agonists. This also includes Wegovy, an injectable drug that is approved for use as a weight loss medication in obese patients.[1]

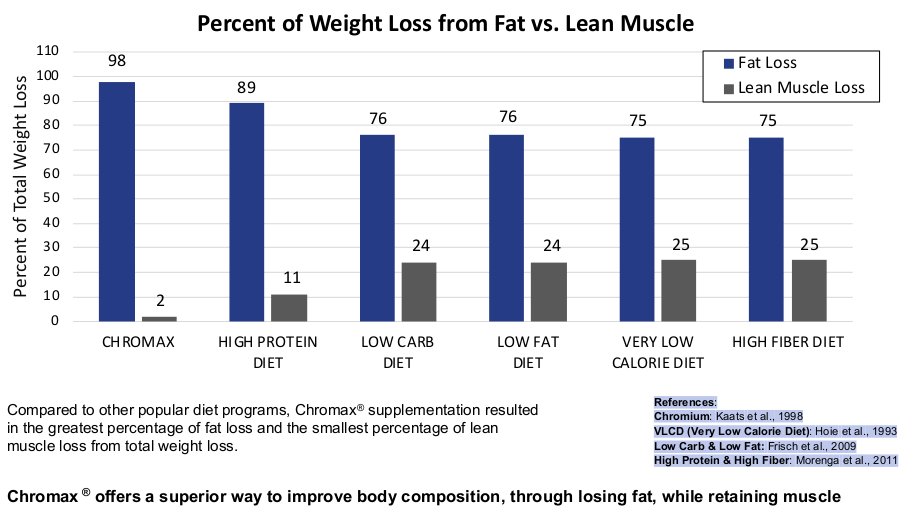
We're entering a new paradigm of dietary supplementation, with a goal of losing fat, not muscle. Regardless of your use of GLP-1 drugs or not, Chromax® Chromium Picolinate has a great place in every healthy person's life!
While these medications show promise for weight loss, they're not a panacea, and dieters should always consider all health avenues, whether or not a GLP-1 agonist drug is used. This includes diet, exercise, and supplements to fill nutritional gaps.
Savvy consumers and dietary supplement brands must keep this in mind when developing supplement stacks and products to support weight and health goals -- users of GLP-1 medications represent a new and growing demographic in the market to assist.
Consider Chromax®
In this article, we explore one ingredient that should be considered in supplement stacks and formulations: Nutrition21's Chromax®, which is the industry's premier form of chromium (as chromium picolinate). Chromax has demonstrated an ability to:
- Help maintain muscle when losing weight and support overall body composition
- Support carbohydrate metabolism and insulin function, and
- Help manage cravings and appetite.
Regardless of a dieter's decision to take a pharmaceutical approach to weight loss, these are all great benefits to pursue.
Let's look at some of the recent research on these drugs, and see if there's a connection to be made with chromium supplementation:
2024 Research review: Semaglutide and Lean Mass Loss
The news surrounding GLP-1 agonists has highlighted many of their positive and negative effects, reinforcing the stance that diet, exercise, and supplementation need to be considered in all situations.
In recent weeks, new reports of Ozempic's side effects have emerged, which can be summarized succinctly by reference to the Atlantic's headline proclaiming that "Ozempic Makes You Lose More Than Fat".[2] Unfortunately, it seems that treatment with this drug can cause significant amounts of muscle loss, as explored by an April 2024 systematic research review investigating six separate studies.[3]
Still – the same review found that even in larger trials where the reduction in lean mass was more evident, semaglutide treatment increased the study subjects' proportion of lean mass, indicating a net improvement to overall body composition.[3]
Whether this makes Ozempic "worth it" for any given dieter is highly dependent on that person's individual context. Some people can maintain a high-protein diet with resistance training, which may stave off many of these effects. Others also have more muscle mass to begin with. So, as always, you should ask your doctor if these findings may relate to you.
GLP-1 Agonists aren’t going anywhere, but there are issues to consider
Ozempic has become a handy signifier for the entire category of drugs, which is based on its primary mechanism of action, glucagon-like peptide 1 (GLP-1) agonism. These are drugs that increase the expression of GLP-1, which enhances insulin secretion, improves blood glucose control, and promotes satiety by slowing gastric emptying,[4-9] thereby aiding in the management of diabetes and obesity.
With the improvement of satiety comes the reduction of appetite, and thus, food intake. This can lead to nutritional gaps, and that's exactly where dietary supplements fit in -- from protein to fiber to essential minerals.
The question becomes, how can we support dieters' effectiveness and prevent issues from reduced food consumption?
-
Keep protein high
Powered by Nutrition21's Velositol, Podium Nutrition's HWPO Whey Isolate Protein makes hard work pay off even more!
The first idea that comes to mind is protein and essential amino acid supplementation. Since high-protein diets have been shown to support body composition with nearly every dietary tactic,[10-14] it's imperative to keep the critical macronutrient high, support as much muscle mass as possible. Protein shakes can certainly support these goals.[15-19]
For example, a protein powder we've recently covered that keeps the fats, calories, and carbs lower with the protein still high is Podium Nutrition's HWPO Whey Isolate. Even someone whose appetite is a bit lower can appreciate a quick shake like this, keeping amino acids circulating, supporting muscle tissue.
-
Don’t forget the healthy fats
Dieters with lower appetites are likely to be dropping added fats, as well. This is a great opportunity to re-balance the omega-3:omega-6 ratio, which was historically closer to 1:1 before the industrialization of food.[20-23] Higher omega-6 ratios are predictive of cardiovascular disease,[20,24] insulin resistance and metabolic dysfunction,[25-28] diabetes,[29-31] and obesity.[32-34]
So the goal here is to reduce the industrial omega-6 fats, and boost omega-3 fatty acids, which have been shown to support critical metabolic biomarkers.[35-39]
-
Consider fiber
Fiber supplementation is often situation-dependent, but in general, there are an incredible number of benefits to its supplementation.[40]
Aside from supporting the gut microbiome,[41] fiber has been shown to improve insulin sensitivity and overall metabolic health improvements[42] while supporting healthy weight and abdominal fat reduction.[43,44] It also improves gut motility and prevents constipation,[45] which could greatly help this new generation of dieters.
However, not everyone responds incredibly well to fiber, so it's worth putting into a separate formula and allowing the right consumers to use it.
-
Resistance train!
We'd also be remiss to mention resistance training, which supports the development and maintenance of skeletal muscle, especially when in a positive nitrogen environment (eg. keep protein intake high).[46] Dieters don't need to be breaking world records or accomplishing Olympic lifts, but they should work to get their muscles to respond to stimulus.
Beyond the initial ideas of protein and fiber, however, vitamin and mineral intake may not be adequate, and this is where supplementation comes in. And one mineral in particular aligns extraordinarily well with diet.
Chromax Chromium – Great For Diet, Health, and Body Composition
Whenever anyone reduces their overall caloric intake -- whether it's from GLP-1 use or not -- there are additional steps beyond protein intake that should be considered. A prime candidate is Chromax (chromium picolinate) from Nutrition 21.
-
Supports insulin signaling
GLP-1 agonists are insulinogenic, which is actually not always a bad thing. As it turns out, these drugs only increase insulin secretion when blood glucose levels rise, which is why they can help decrease postprandial hyperglycemia without having an adverse effect on baseline insulin levels.[47]
For over 25 years, Chromax chromium picolinate has been improving insulin sensitivity. We argue that it's only gotten better, as dietary mineral deficiencies have gotten worse over this time period.
Still, in order to optimize metabolic health, we want the body to use insulin as efficiently as possible, and that's where Chromax comes in.
Ingested chromium binds to a low-molecular-weight peptide complex referred to as low-molecular-weight chromium-binding substance (LMWCr). This complex is composed of several amino acids that can chelate chromium ions, forming the active complex known as chromodulin.[48,49]
Though the exact mechanism through which chromium impacts insulin is still unknown, one mechanism is thought to relate to this chromium-chromodulin interaction. One study found that chromodulin activates the insulin receptor,[50] in a manner that roughly approximates insulin itself – but it also amplifies the effect of circulating insulin. One in vitro study has found that chromodulin can increase insulin's signaling activity by an incredible 800%.[50] And when it comes to stimulating chromodulin activity, chromium is in a class of its own – there is no other mineral that's been shown to have a similar effect on chromodulin.[51]
Thus, Chromax can potentially lead to a positive effect on promoting a healthy insulin response, and we suspect it will be for numerous types of dieters, regardless of their choice on GLP-1 drugs.
-
Insulin Resistance, High-Sugar Diets, Stress, and Chromium Availability
Data shows that individuals with severe insulin resistance have lower chromium levels, as measured in hair, sweat, and serum.[52] Additional research has found that those with diets high in sugar may have depleted chromium levels, since its excretion is elevated in those dietary circumstances.[53] Other circumstances, such as those who are chronically stressed or exercise too little also have increased excretion of chromium.[54] While not causal, these data suggest a relationship between carbohydrate metabolism and chromium.
-
Appetite Suppression
Other evidence suggests that Chromax could support healthy appetite in dieters.
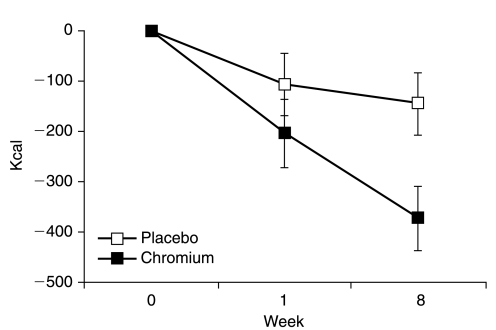
For example, in one randomized, double-blind, placebo-controlled study from 2008, researchers administered Chromax® chromium picolinate to overweight women. For eight weeks, they received either 1,000 mcg of chromium, or a placebo, while each group's caloric intake was tracked.
By the end of the study period, the Chromax® group exhibited significantly less food intake, decreased hunger levels, and more specifically, lower cravings for dietary fat.[55] The Chromax group also showed decreased body weight – while the placebo group gained about a pound of weight, the Chromax group lost about the same amount.[55] While these aren't massive changes in weight, the inclusion of Chromax chromium picolinate helped manage aspects of appetite which may have helped to support some weight loss.
In this study, overweight women who took chromium reduced their food intake by significantly more than the placebo group..[55]
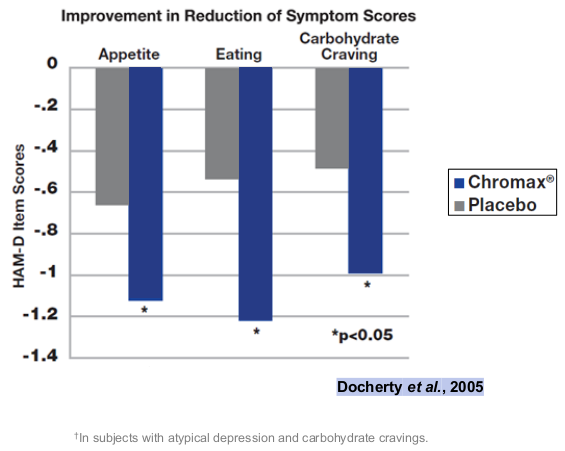
Another, earlier randomized double-blind study, published in 2005, administered either 600 mcg chromium as Chromax chromium picolinate or a placebo to 113 adults with atypical depression. It showed that chromium ingestion was associated with significant reductions in appetite, food intake, and carbohydrate cravings, compared to the placebo.[56]
It's interesting to note that the first study showed lower cravings for dietary fat,[55] while the second showed reduced carbohydrate cravings.[56] Either direction may support the needle for higher protein intake, which is one of our primary goals for health and body composition.
Both of these effects are more heavily covered in our article titled "Chromax and Hunger Regulation: The Chromium / Appetite Connection". Ultimately, managing cravings and appetite while dieting can be very difficult for some -- and Chromax may help.
Why Chromax?
Of course, if a dieter were to consider chromium supplementation, one would want to choose the best form of chromium available, and existing research indicates that the best is Chromax from Nutrition21. Two different peer-reviewed studies have demonstrated that Chromax is as much as 15 times more bioavailable than other types of chromium.[57,58]
Dosage: 1000 micrograms for appetite reduction
In terms of dosage, Chromax has been studied in dose ranges providing 200 micrograms to 1000 micrograms of elemental chromium. The two studies showing reductions in cravings provided 600 and 1000 micrograms of chromium,[55,56] so we generally opt for the higher doses in these applications.
Conclusion: Either Way, Chromax Has a Place
GLP-1 medications have various clinical benefits and have been shown to help manage appetite. Whether taking these drugs or trying to manage your health through diet and exercise alone, chromium supplementation may help dieters achieve their health goals by promoting healthy body composition, supporting nutrient metabolism via effects on insulin, and even by helping manage cravings.
Backed by over 25 years of research, Chromax -- which is made in the USA -- has a great place in the health routines of dieters worldwide.
You can read more in our articles titled "Chromium Picolinate: The Ultimate Trace Mineral For Insulin Sensitivity" and "Chromax Chromium Picolinate: 25+ Years of Insulin Sensitivity Improvement", and sign up for our Nutrition21 news alerts below:





Comments and Discussion (Powered by the PricePlow Forum)