"You'll struggle to find anything else with clinical evidence of cartilage regression."

6-month study shows AprèsFlex delivers MRI-verified cartilage regeneration at just 100mg daily. Not just pain relief -- actual structural improvement. STROM UK's Rick Foster explains why this sets it apart from typical joint supplements.
Rick Foster, founder of STROM UK, didn't mince words during his recent visit to PLT Health Solutions headquarters in Morristown, New Jersey. In a forthcoming episode of the PricePlow Podcast, Rick, along with PLT's Steve Fink and STROM US director (Dean), dove deep into what separates legitimate joint health ingredients from the crowded field of "me-too" supplements.
For those unfamiliar with STROM, this isn't your typical supplement brand. Born from the UK's hardcore training culture, STROM has built its reputation on formulations that work. When Rick talks about needing actual evidence, he means MRI scans, biomarkers, and data that would satisfy the most skeptical strength athlete.
And that's exactly what caught his attention about AprèsFlex.
The conversation at PLT will teach listeners that most joint supplements focus on symptom management (reducing pain, improving mobility), but AprèsFlex does something fundamentally different. It addresses the underlying structural problem. We're talking about actual cartilage regeneration visible on medical imaging.
The new six month study is covered in this article. Before we dig in, subscribe to our PLT Health news alerts for clinical research updates, new ingredient launches, and science-backed formulation insights:
Subscribe to PricePlow's Newsletter and Alerts on These Topics
The Cartilage Regression Claim: What Makes AprèsFlex Different
When Rick mentioned "cartilage regression", he's referring to something most joint ingredients can't touch: actual reversal of cartilage degradation. Not just slowing it down. Not just maintaining what you have. Actually improving the structure of damaged cartilage tissue.
Pain reduction is table stakes -- glucosamine and chondroitin can already do that a bit. But structural improvement? That requires a different level of clinical validation.
The evidence Rick referenced comes from a recently published 180-day clinical trial that used advanced MRI imaging to track changes in knee cartilage.[1] The study provided visual, measurable proof of cartilage improvement.
And here's the kicker: all of this happens at just 100mg daily, with benefits starting as early as five days.
Let's get into it:
The Kumar 2024 Study: Six Months of MRI-Verified Joint Regeneration
The Kumar study represents the kind of clinical research that budget-based companies often avoid: it's expensive, time-consuming, and requires genuine confidence in your product.[1]
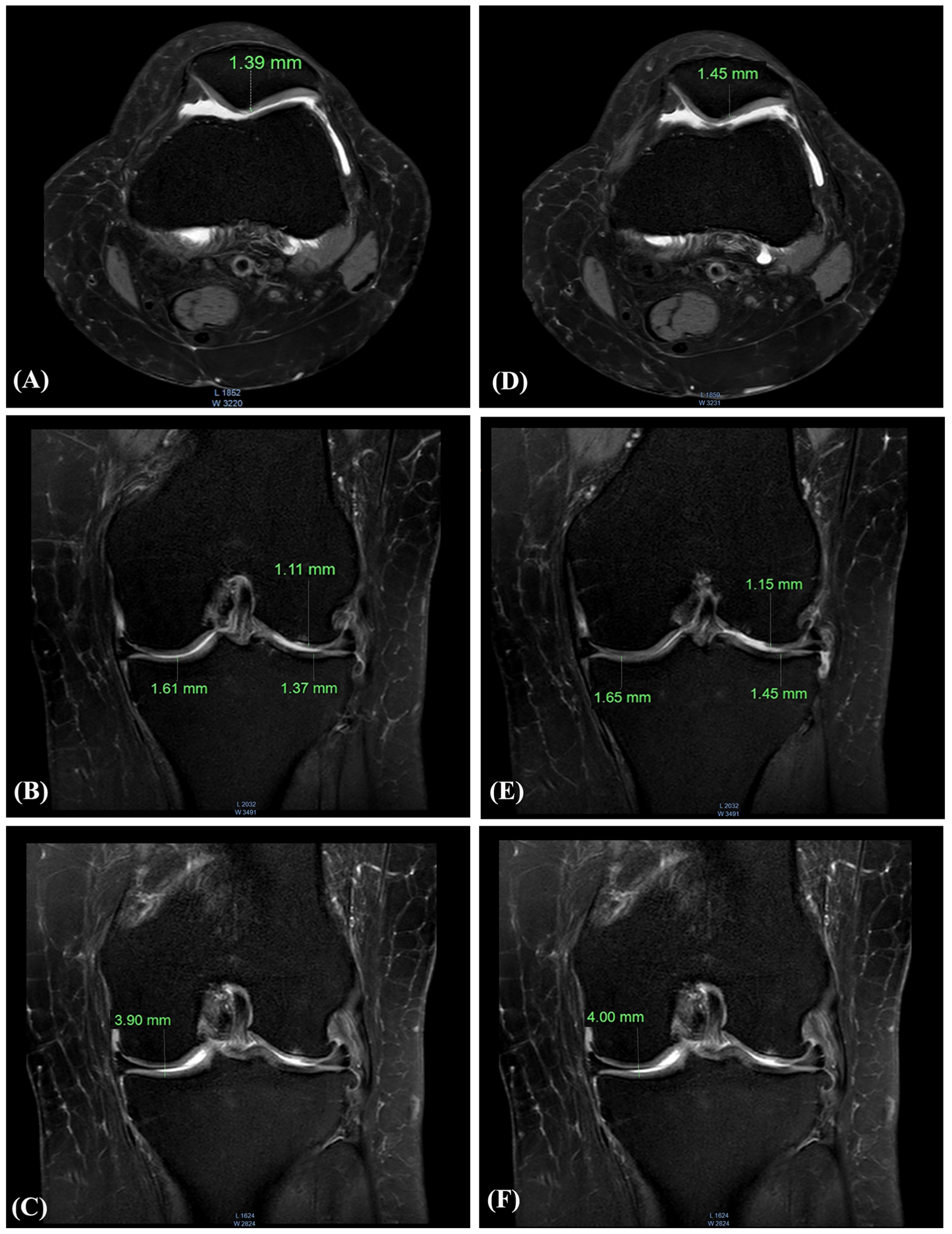
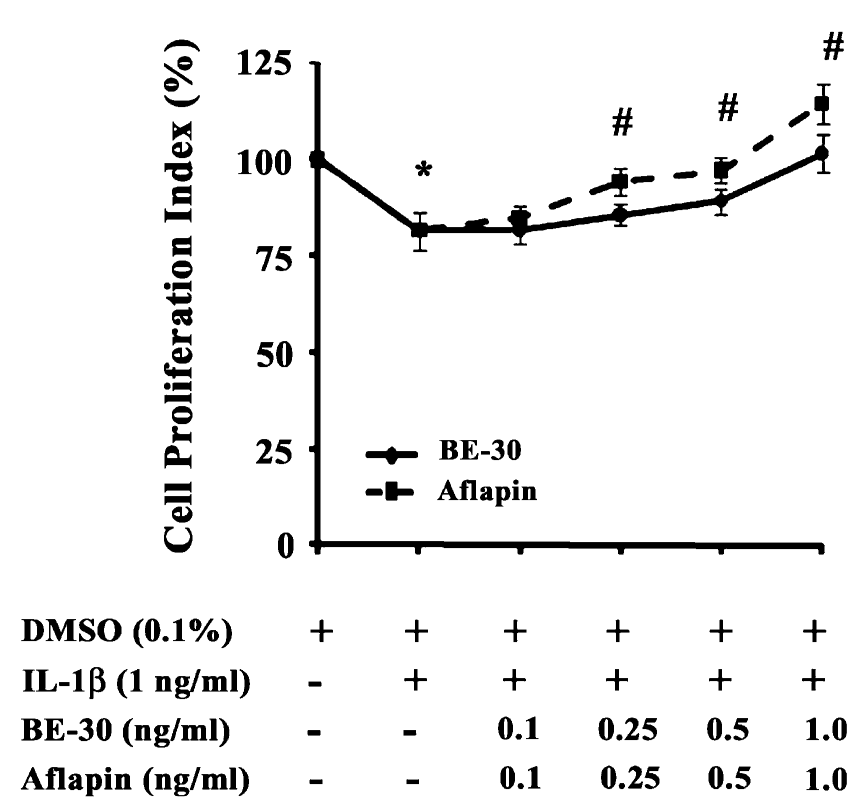
These MRI scans from a 47-year-old female participant show measurable increases in knee cartilage thickness after six months of daily AprèsFlex supplementation. The imaging reveals improvements in both the medial and lateral compartments of the knee, along with increased joint space width.[1]
Eighty participants with mild-to-moderate knee osteoarthritis (Kellgren-Lawrence grades II-III) were randomly assigned to receive either 100mg of AprèsFlex or placebo daily for 180 days. That's six months!
The imaging protocol used a state-of-the-art 3-Tesla MRI scanner with specific parameters designed to capture detailed cartilage morphology. Two independent radiologists, each with over ten years of musculoskeletal imaging experience, analyzed the results while blinded to treatment assignment. The interclass correlation coefficient between readers ranged from 0.82 to 0.92, indicating high reliability of the measurements.
The Visual Evidence: MRI Results That Speak Volumes
The MRI analysis revealed improvements across multiple cartilage parameters in the AprèsFlex group compared to placebo. Participants taking AprèsFlex showed measurable increases in:[1]
- Cartilage thickness in the medial and lateral tibial plateaus
- Cartilage volume throughout the knee joint
- Joint space width between the femur and tibia
The study included side-by-side MRI comparisons showing baseline versus day-180 measurements. For one 47-year-old female participant, medial tibial cartilage thickness increased from 1.37mm to 1.45mm, while lateral measurements improved from 1.61mm to 1.65mm. Joint space width expanded from 3.90mm to 4.00mm.
These may seem like small numbers, but in the context of osteoarthritis, a condition characterized by progressive cartilage loss, any increase is remarkable. Most interventions aim to slow degradation. AprèsFlex appears to support regeneration.
Pain and Function: The Numbers Patients Feel
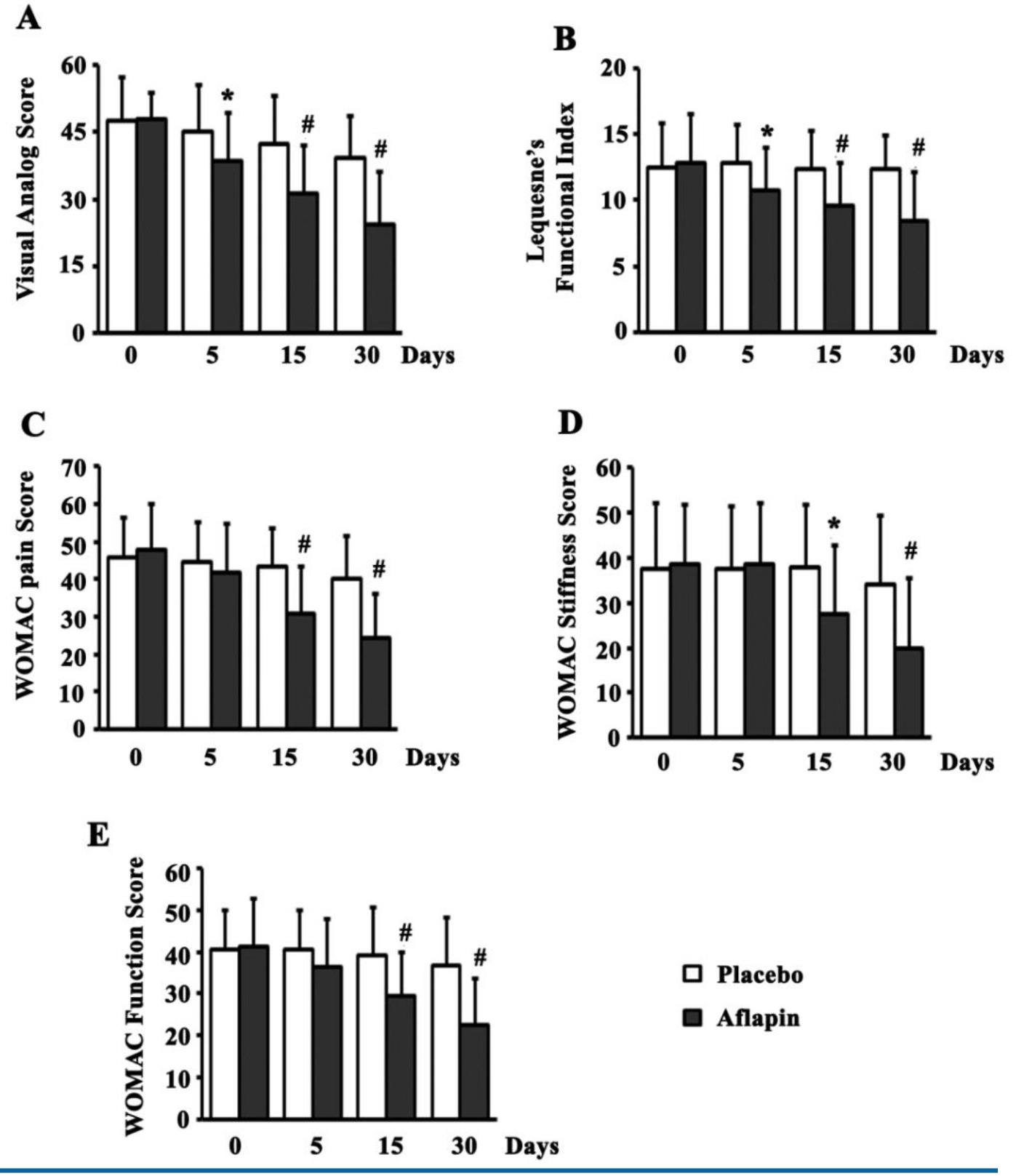
The rapid-onset study confirmed that AprèsFlex begins delivering measurable benefits within just five days of supplementation. Participants experienced progressive improvements in pain levels, joint stiffness, and physical function throughout the 30-day trial period.[2]
While structural improvements matter from a scientific perspective, participants cared most about one thing: how they felt.
The results delivered on multiple validated assessment scales:[1]
- WOMAC Scores (Western Ontario and McMaster Universities Osteoarthritis Index) improved significantly across all three subscales:
- Pain scores decreased throughout the study period
- Stiffness measurements improved consistently
- Physical function assessments showed progressive benefits
- Visual Analog Scale (VAS) pain ratings dropped substantially in the AprèsFlex group compared to minimal changes with placebo. Participants reported meaningful reductions in knee pain during daily activities.
- Lequesne's Functional Index, which measures pain, walking distance, and activities of daily living, showed consistent improvement. Participants found it easier to perform tasks that had been challenging or painful before the study.
- Physical Performance Tests backed up the subjective reports:
- The six-minute walk test showed participants could cover more distance
- Stair climb tests revealed faster ascent and descent times
- Both measurements improved progressively throughout the 180 days
Biomarker Breakthroughs: The Biological Mechanisms
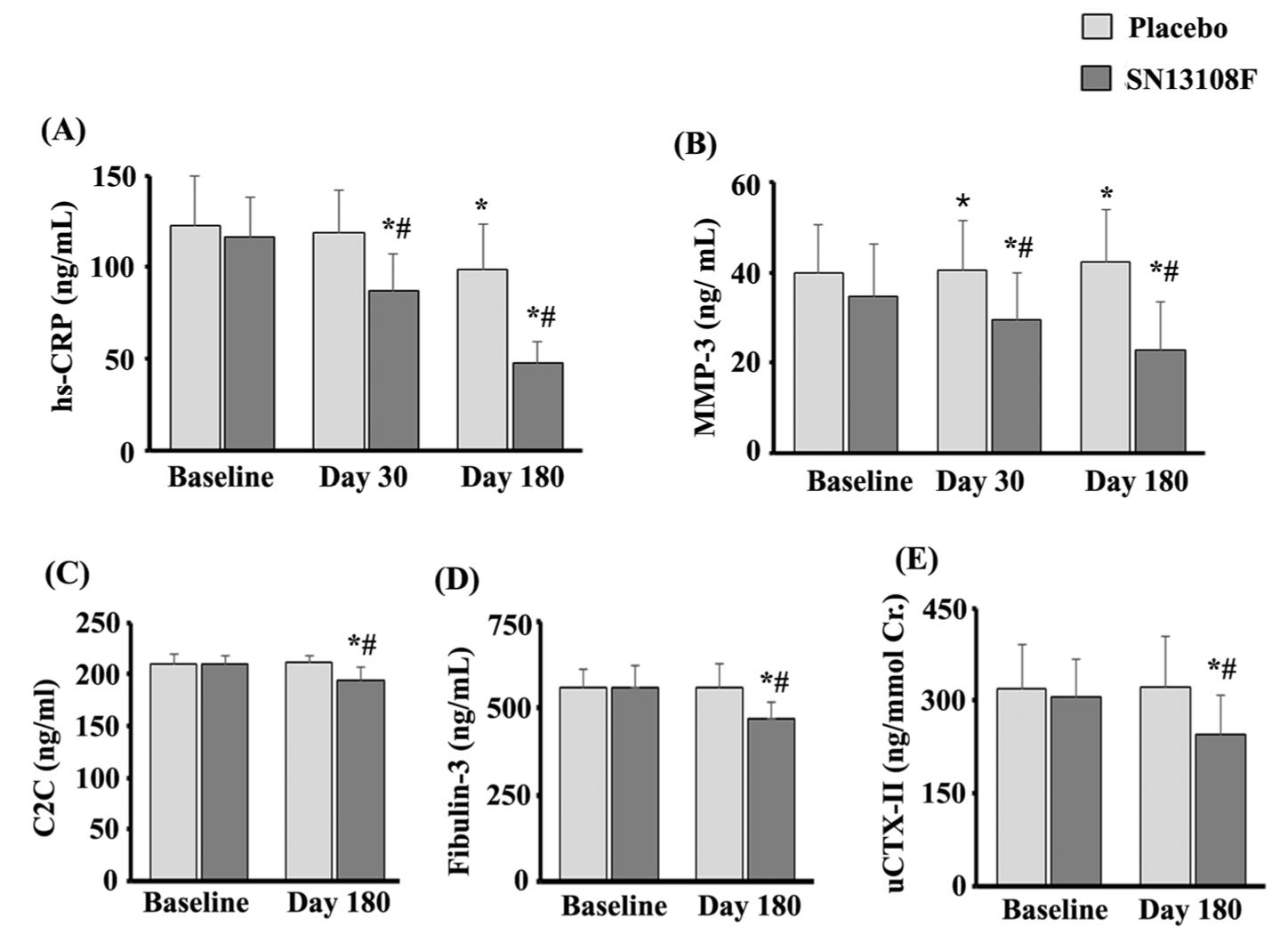
Blood and urine tests revealed favorable changes in key joint health biomarkers throughout the six-month study. The AprèsFlex group showed reductions in inflammation markers and cartilage breakdown indicators compared to placebo, suggesting genuine biological improvements rather than just symptom masking.[1]
Perhaps most interesting from a mechanistic perspective were the biomarker results, which revealed how AprèsFlex works at the molecular level.[1]
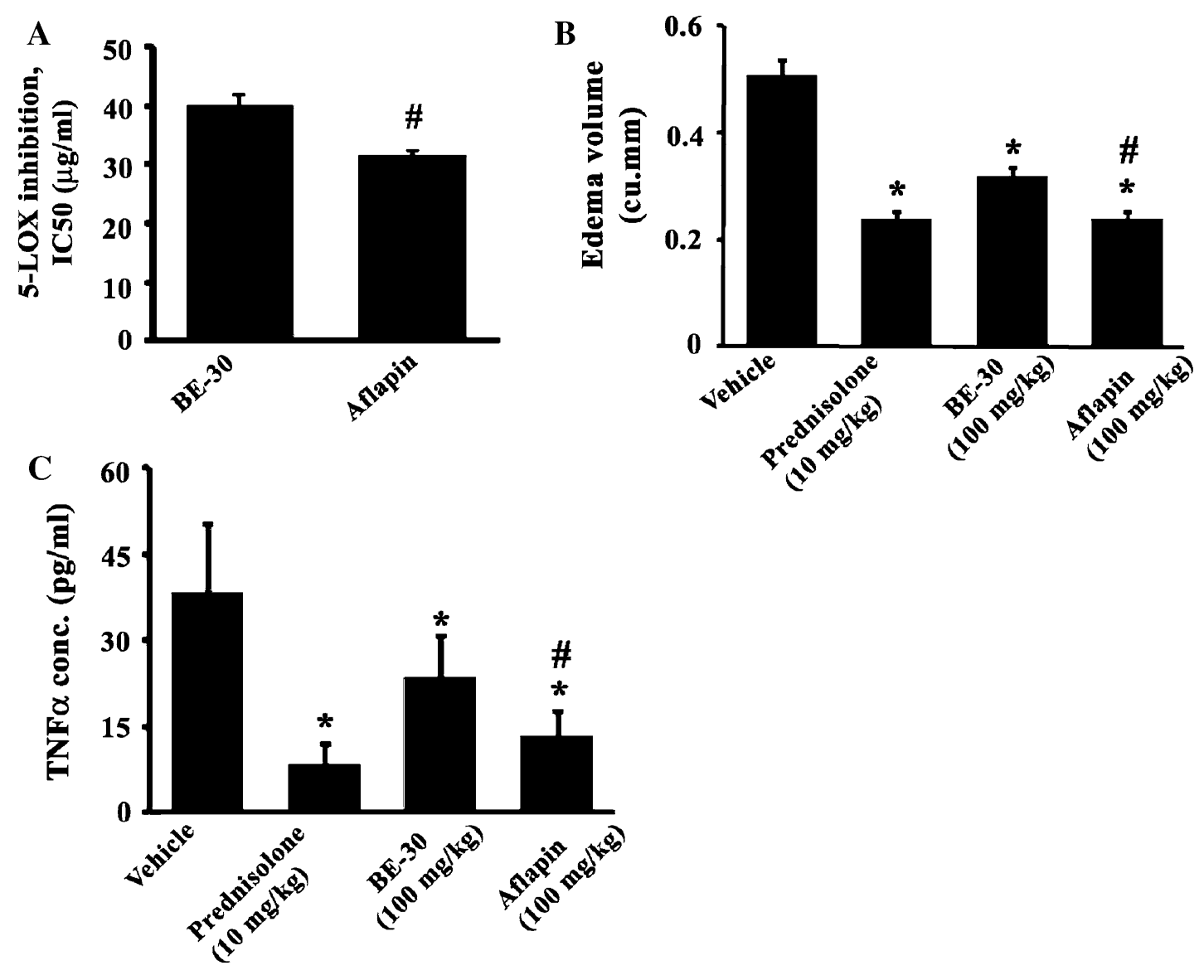
Laboratory and animal studies demonstrate AprèsFlex's ability to inhibit inflammatory pathways, reduce swelling, and lower pro-inflammatory signaling molecules. These preclinical findings help explain the clinical benefits seen in human trials.[3]
- Matrix Metalloproteinase-3 (MMP-3), an enzyme that breaks down cartilage, collagen, and connective tissue, decreased in the AprèsFlex group. This is critical -- reducing MMP-3 means less cartilage destruction occurring in the joint.
- High-sensitivity C-Reactive Protein (hs-CRP), a marker of systemic inflammation, dropped in participants taking AprèsFlex. Lower inflammation creates a better environment for tissue repair and regeneration.
- Urinary CTX-II (cross-linked C-terminal telopeptide of type II collagen), a biomarker of cartilage degradation, decreased by 23.63% in the AprèsFlex group while the placebo group saw a slight increase. This marker directly correlates with cartilage breakdown: less CTX-II means less cartilage being destroyed.
The combination of reduced cartilage-degrading enzymes, lower systemic inflammation, and decreased cartilage breakdown markers explains the structural improvements seen on MRI. AprèsFlex appears to create a biological environment conducive to cartilage preservation and regeneration.
Safety Profile: Built for Long-Term Use
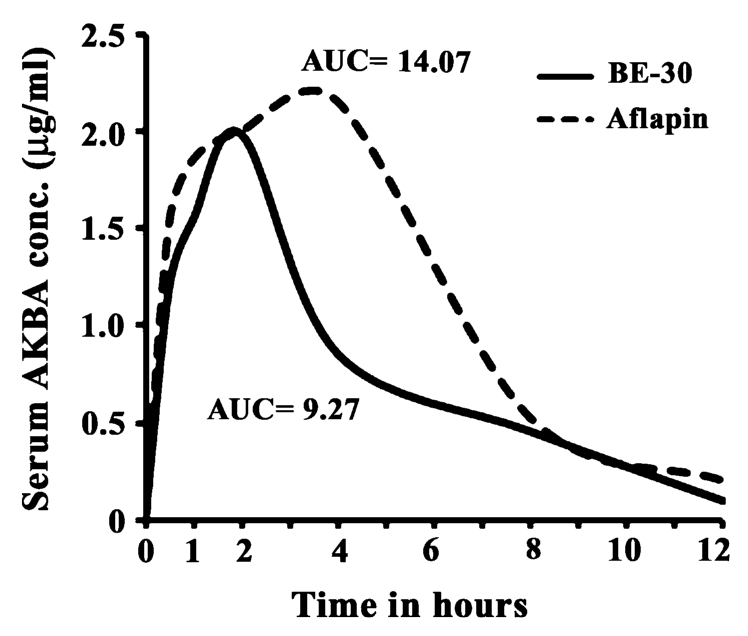
AprèsFlex delivers superior absorption of AKBA, the active compound in Boswellia, compared to standard extracts. The enhanced bioavailability means more of the beneficial compound reaches the bloodstream where it can support joint health.[3]
One concern with any daily supplement is long-term safety, especially for a condition like osteoarthritis that requires sustained intervention.
The Kumar study found zero product-related adverse events related to AprèsFlex supplementation over 180 days.[1] Comprehensive blood work, including hematology and complete serum biochemistry panels, remained within normal ranges throughout the trial. Vital signs showed no concerning changes.
Previous toxicology studies in animal models established a no observed adverse effect level (NOAEL) greater than 2,500mg/kg body weight in rats, providing an enormous safety margin considering the 100mg human dose.[4]
This safety profile matters for formulators creating products intended for daily, indefinite use by aging populations managing chronic joint conditions.
The Supporting Science: A Portfolio of Clinical Evidence
The Kumar 2024 study represents the pinnacle of AprèsFlex research, but it builds on a foundation of earlier investigations that established the ingredient's efficacy and mechanisms.
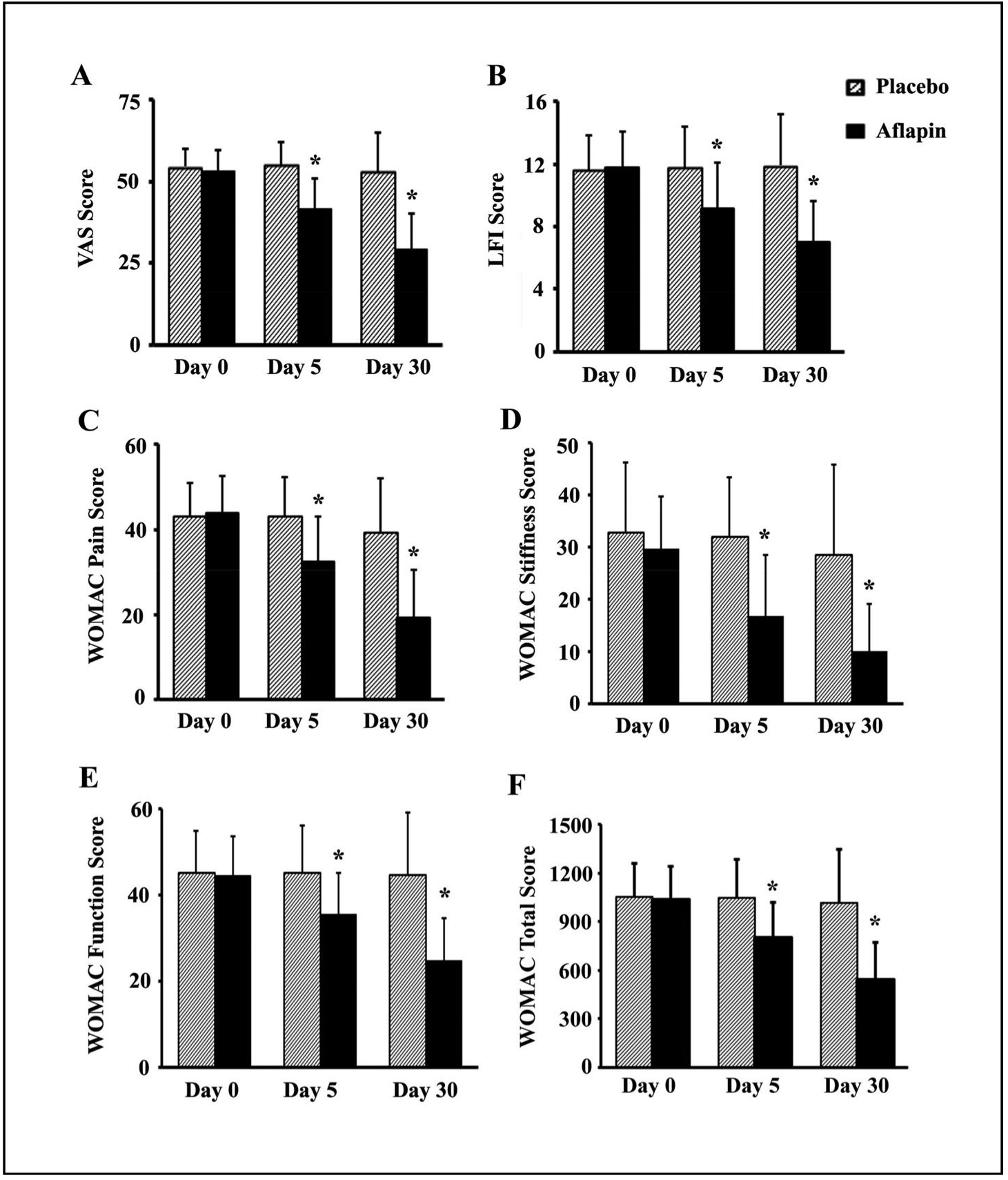
Participants taking 100mg of AprèsFlex daily experienced significant improvements across multiple joint health metrics within just 30 days. The treatment group showed better outcomes in pain reduction, mobility, and daily function compared to placebo across all validated assessment tools.[5]
The 5-Day Onset Study: Fast-Acting Joint Relief
A 2022 clinical trial examined whether AprèsFlex could deliver benefits quickly enough to satisfy consumers expecting rapid results.[5] The results exceeded expectations: participants taking 100mg of AprèsFlex showed significant reductions in pain scores at just five days compared to placebo. By day 30, the benefits had expanded to include substantial improvements in physical function and mobility. This rapid onset addresses a key challenge in joint health supplementation: customers often abandon products before they have time to work. AprèsFlex demonstrates measurable benefits within the first week, improving adherence and satisfaction.
The Original Validation: Establishing the Effective Dose
The Vishal 2011 study provided early validation of AprèsFlex's joint comfort benefits and established that 100mg represents an effective daily dose.[2] This research laid the groundwork for subsequent investigations by confirming that relatively low doses of this AKBA-enriched Boswellia extract could deliver clinical benefits. The study's success prompted deeper mechanistic investigations and longer-term trials.
The Mechanism Study: How AprèsFlex Works
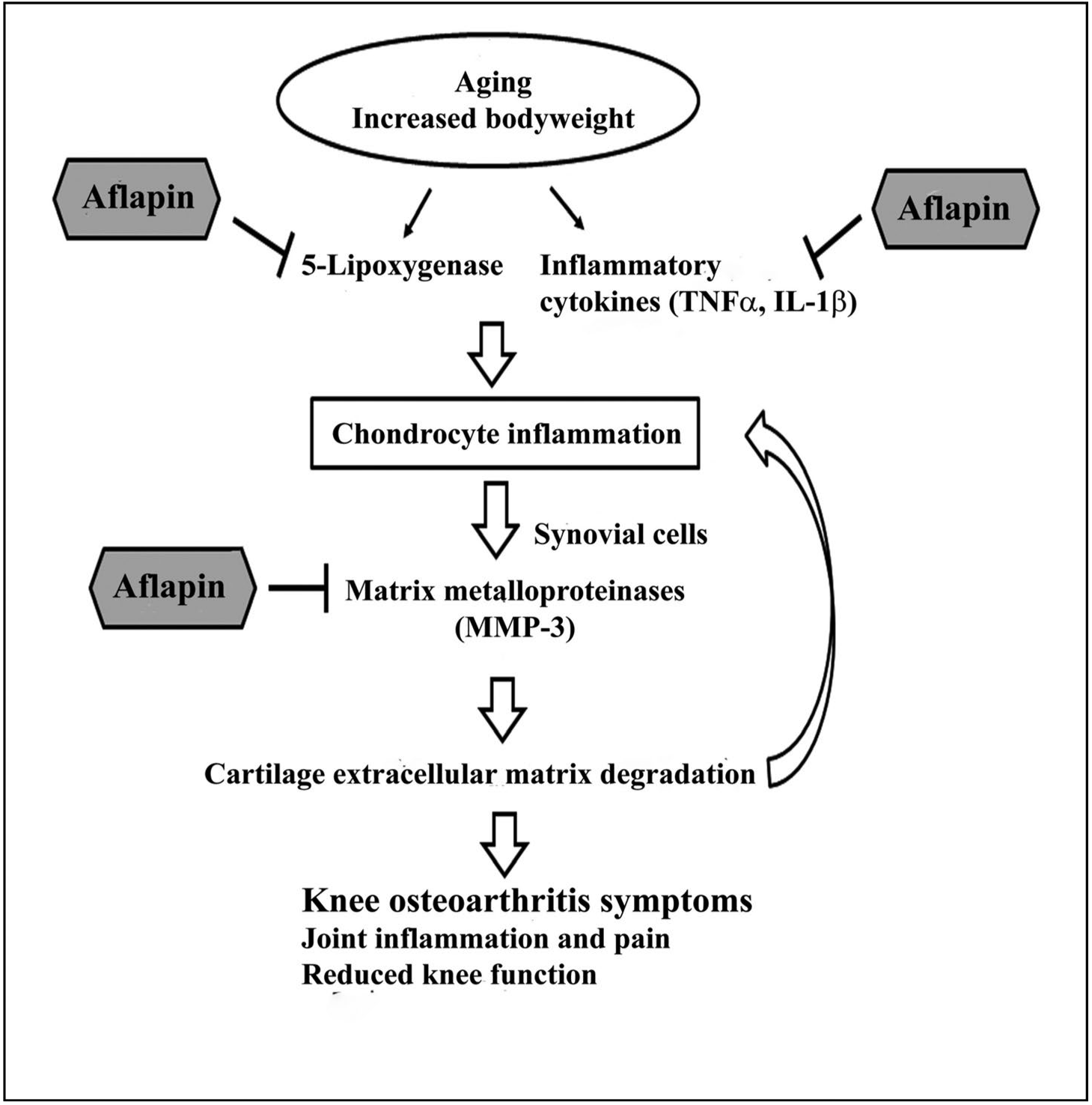
AprèsFlex works through multiple pathways to support joint health. The ingredient targets inflammatory enzymes and signaling molecules that contribute to cartilage breakdown, helping to create a better environment for joint tissue maintenance.[5]
Understanding why an ingredient works matters for formulators making strategic decisions about combinations and applications. Research into AprèsFlex's cellular mechanisms revealed that the AKBA-enriched extract inhibits 5-lipoxygenase activity, reducing production of pro-inflammatory leukotrienes.[3] The extract also reduces levels of inflammatory cytokines and matrix metalloproteinases in cartilage cells while protecting glycosaminoglycans—the building blocks of healthy cartilage. This multi-targeted approach explains why AprèsFlex can simultaneously reduce pain, lower inflammation, and support structural improvements.
Why STROM Chose AprèsFlex: The Formulator's Perspective
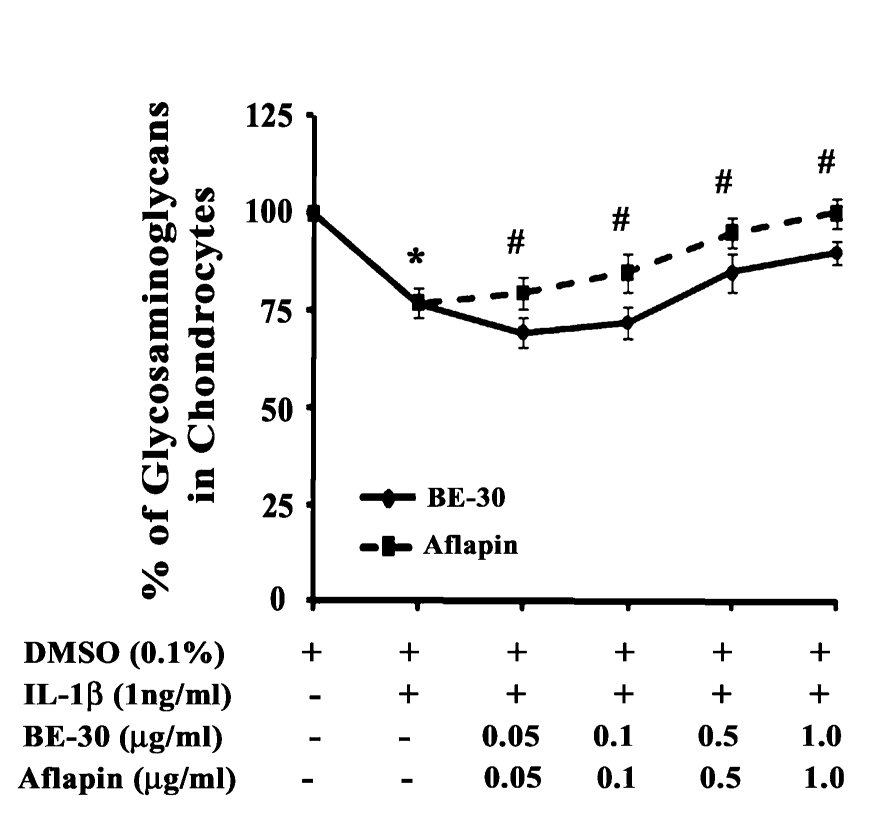
AprèsFlex demonstrated the ability to support production of glycosaminoglycans, essential structural components of healthy cartilage. This activity helps explain how the ingredient may contribute to cartilage regeneration seen in clinical imaging studies.[3]
During the podcast discussion at PLT HQ, Rick explained his approach to ingredient selection. STROM's reputation rests on formulations that work for serious strength athletes -- people who can instantly tell if a product delivers. But Rick also applies "the parent test": would this work for his mother? This philosophy ensures products satisfy both hardcore users and aging adults seeking mobility.
For Beast Farm Big Joint, AprèsFlex fit perfectly. The clinical evidence appealed to informed consumers who research ingredients, while the rapid onset worked for regular users wanting relief. Rick valued the cartilage regression evidence because UK consumers increasingly verify ingredient claims through social media and research tools.
Dean emphasized that American consumers follow a similar trajectory: demanding science-backed branded ingredients over generic formulations. The combination of AprèsFlex with complementary ingredients like HydroCurc creates synergistic anti-inflammatory effects.
The Bigger Picture: Joint Health Evolution
In laboratory studies, AprèsFlex helped cartilage cells maintain healthy growth even when exposed to inflammatory stress. This cellular-level support may contribute to the ingredient's ability to promote joint tissue health in humans.[3]
The joint health category is evolving beyond glucosamine, chondroitin, and MSM. Informed consumers now seek ingredients backed by human clinical trials with objective measurements like MRI imaging, rapid onset of benefits, and safety data for long-term use. AprèsFlex delivers on all fronts with unusually strong evidence.
This evolution parallels broader wellness and biohacking trends. Younger athletes think proactively about joint health rather than waiting for problems to develop. The longevity movement amplifies this shift—people optimizing for health span want to preserve function and mobility for decades, requiring ingredients that support underlying structures rather than masking symptoms.
What's Next: More from the PLT HQ Discussion
The conversation at PLT Health Solutions covered far more than AprèsFlex. Rick, Dean, and Steve discussed UK versus US supplement regulations, novel ingredient challenges, pre-workout evolution, and cognitive enhancement supplements. When the full episode drops, you'll hear Rick's take on proprietary blends, Dean's perspective on D2C customer acquisition, and their debate about whether hardcore consumers actually care about flavor.

Dive into PLT Health's cognitive platform with Steve Fink and Dr. Jeremy Appleton, exploring Zynamite, Zembrin, and Vanizem's impact on brain health in Episode #146 of the PricePlow Podcast
The Bottom Line
AprèsFlex stands apart in the joint health category because it delivers visual, measurable evidence of cartilage improvement... not just symptom relief. The Kumar 2024 study's MRI results provide the kind of proof that satisfies both skeptical formulators and informed consumers.
For Rick and STROM, this level of validation matters because their reputation depends on formulating products that actually work. Beast Farm Big Joint needed ingredients that could deliver benefits for both hardcore strength athletes and the general population seeking better joint health.
The 100mg effective dose, five-day onset of benefits, and comprehensive safety profile make AprèsFlex an ideal choice for formulators creating joint health products intended for long-term daily use. The combination of rapid symptom relief and sustained structural improvements addresses both immediate customer satisfaction and long-term efficacy.

PLT Health Solutions: Growth Through Innovation
As supplement consumers become more sophisticated and demand genuine clinical validation, ingredients like AprèsFlex -- backed by MRI imaging, biomarker analysis, and rigorous human trials -- will increasingly separate legitimate products from marketing hype.
Don't miss the full STROM UK podcast discussion when it drops on the PricePlow Podcast, featuring deep dives into ingredient selection, UK vs. US market differences, and the future of evidence-based supplementation.
Subscribe to the PricePlow Podcast on Your Favorite Service (RSS)
For ongoing PLT Health ingredient updates and research: Check the PLT Health news section on PricePlow or subscribe using the form below:





Comments and Discussion (Powered by the PricePlow Forum)