While nobody should ever accept mediocrity, there are certain times where trust and perfection are of absolute critical importance. One of those times is with liver health, especially in the bodybuilding community. On top of doctor-directed lab testing and care, dietary supplements can play a major role in hepatoprotection.
When it comes to trusted and tested dietary supplements, one of the first names to come to mind is always NutraBio. Led by industry pioneer and trend-setter Mark Glazier, the brand has lab-tested every single lot manufactured since 2017. And when considering TUDCA, the ingredient discussed in great detail below, there's no greater time to get lab tests.
Reason being, this incredible liver-protective ingredient has been difficult to source and has been subject to online scams. This is one time where we need to be 100% positive that what you're getting in the bottle is what's on the label, because some bodybuilders may have catastrophic consequences without it. This ingredient is that important.
NutraBio TUDCA: Trusted and Tested, front and back

NutraBio is the industry leader in quality and transparency, and has been for quite some time.
We recently covered the numerous benefits of milk thistle and the importance of proper extracts and third-party testing in our NutraBio Milk Thistle article. Today, we do it again with NutraBio TUDCA, diving very deep into TUDCA, which has had a lot of recent research covering more than just liver health.
As we know from The NutraBio Story, NutraBio has fully disclosed supplements and follows the letter of the law, testing incoming raw materials, outgoing products, and they even publish 3rd party lab tests online. When you absolutely need it to be done right, you need NutraBio.
Let's check PricePlow's coupon-powered prices and get into the latest research on this fascinating compound:
NutraBio Tudca – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.
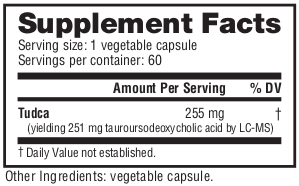
NutraBio Tudca Ingredients - 251 milligrams TUDCA
NutraBio TUDCA contains 251 milligrams of pure, third-party lab tested TUDCA, which is short for Tauroursodeoxycholic acid.[1,2] TUDCA is a taurine conjugate of ursodeoxycholic acid, known as UDCA. While UDCA is an FDA-approved drug for the treatment of biliary cholangitis (previously primary biliary cirrhosis), the taurine form in TUDCA is sold as a dietary supplement.
And it turns out that TUDCA works better than UDCA for the vast majority of use cases, including the ones that most aggressive athletes care about!
A bit on bile acids
The story begins with Traditional Chinese medicine, where bear bile was used to treat numerous disorders (such as retinal degeneration and hepatic [liver] disorders), TUDCA is a primary constituent of bears' bile.[3,4]
Bile acids are products of cholesterol breakdown, and the associated biliary system is immensely important for countless metabolic functions, especially in terms of the metabolism of dietary fats and fat-soluble vitamins, as well as their absorption and transport.[5]
Normally, the body can synthesize UDCA from cholesterol-only primary bile acids like chenodeoxycholic acid (CDCA) and cholic acid, which go through a series of reactions and gut bacterial processing to form secondary bile acids like UDCA. It can follow numerous other reactions to form TUDCA naturally, but it's a very metabolically-expensive process that may have several bottlenecks along the way.[5]
When our biliary system is not functioning properly, there can be catastrophic consequences, ranging from digestive problems to serious hepatobiliary diseases.[2,6] Thankfully, we can ingest more bile acids to improve liver health (and far more, as the scientific community is recently realizing) while working to treat the underlying condition.
UDCA: The original treatment for cholestasis
Cholestasis is a serious condition that can occur when bile ducts are physically blocked, impairing the flow of bile acids due to their obstruction.[7] This is a grave condition, as inhibited bile production and flow can reduce the digestion of fats, metabolism and transport of fat-soluble vitamins, and ultimately lead to jaundice and destroyed liver tissue.[8] Left unchecked, that can lead to total organ failure. It also turns out that various drugs used by the bodybuilding community can cause cholestasis.[9]
There's good news, though: UDCA has been an extremely helpful treatment for not just cholestasis, but a wide range of hepatic disorders including pediatric cholestatic disorders, primary biliary cholangitis (PBC), and drug-induced cholestasis,[10-17] the last of which is likely our readers' biggest concern.[16,18]
Additionally, UDCA has successfully helped with nonalcoholic steatohepatitis[17,19] and
But we're not here to talk about UDCA, we're here to talk about TUDCA! And it turns out that TUDCA supplements have actually worked better than UDCA drugs in numerous studies:
TUDCA: The Taurine Conjugated form of UDCA
When taurine is bound to UDCA and you get TUDCA, several biochemical properties change, and nearly all of them seem for the better in terms of hepatic protection and rescue.
A landmark multi-center, double-blind trial published in 2016 demonstrated that TUDCA had the same level of safety and tolerability than UDCA, but with greater success in some treatments.[20] In that study, 199 subjects with primary biliary cholangitis (PBC) were randomly put in two groups:
- 250 mg TUDCA plus UDCA placebo, or
- 250 mg UDCA plus TUDCA placebo
Both used three times per day for 24 weeks.[20]
The researchers concluded the following:
"TUDCA is safe and as efficacious as UDCA for the treatment of PBC, and may be better to relieve symptoms than UDCA."[20]
Added to an earlier trial showing that 750 milligrams per day of TUDCA was safer and outperformed UDCA,[21] it was then off to the races for TUDCA-based research. As we'll cover below, there are many other things it can help treat outside of hepatic conditions.
Why does TUDCA work better?

You want high-quality liver supplements, such as the European dry Milk Thistle extracts that NutraBio is using!
The exact mechanisms aren't completely understood, likely because there are several factors simultaneously involved.[2] However, research has shown that the intestines and liver absorb TUDCA better than UDCA because it's fully ionized and water soluble at a greater range of pH values.[21]
In addition, it's known that bile acids become less cytotoxic when they're more hydrophilic ("water loving" - they transfer, travel, and mix better with water). TUDCA's conjugation of UDCA with taurine makes it more polar, which leads researchers to account its "higher therapeutic effectiveness".[2,21-23]
With these effects, TUDCA is well-known for "chaperoning activity" -- it's in a group of biochemical compounds known as chemical chaperones, which have numerous potential benefits in combating endoplasmic reticulum stress.[24-26]
Either way, the exact biochemical mechanism doesn't matter, the end result is that TUDCA ultimately exerts anti-cholestatic properties by enhancing the hepatocytes' secretory capacity.[2,27]
The underlying chemical mechanisms can be further explored by reading the 2019 review article published in Cell titled "Tauroursodeoxycholate—Bile Acid with Chaperoning Activity: Molecular and Cellular Effects and Therapeutic Perspectives"[2]
Bile acids work orally!
In general, bile acid administration is useful because there are many ways they can be used - including oral, subcutaneous, and intravenous administration routes.[2,26] In many cases, they also present low toxicity and can cross the blood-brain barrier,[26] which will lead to additional benefits discussed next.
Other benefits of TUDCA

Joints not working as well as you wish they would? Get this in your stack and feel the difference! Adding TUDCA could make for a very interesting joint support experience.
With the discovery that TUDCA supplementation is so effective (and usually more effective than UDCA), researchers began looking at other potential benefits from its mechanisms.[26] Given that liver health, fat digestion, and fat-soluble vitamin transport are so important to countless health systems, there are a lot of conditions to look at.
Below is just a quick list of benefits from TUDCA, and if you're reading this years in the future, there's a great chance that even more benefits have been discovered since this article was published:
- Protection from and assistance in fighting neurodegenerative disorders[28-33]
- Slows retinal degeneration,[4,34] which is unsurprising since it can improve photoreceptor cells in the retina[35-37]
-
Numerous cytoprotective benefits, including the inhibition of apoptosis pathways,[8] improved cellular stress response,[38,39] mitochondrial membrane stabilization,[40,41] prevention of DNA damage,[42] and antioxidant stimulation.[43,44]
- Osteoarthritis support[45]
The last of which leads us to wonder if there's promise for TUDCA in joint supplements, so this could be very uniquely stacked with NutraBio Curcumin Advanced.
In addition, we've seen long-term general hepatoprotection in rats,[46] leading us to believe that we don't need TUDCA to "treat" anything, but it can definitely be a part of a very solid daily health stack alongside the NutraBio MultiSport Multivitamins.
And of course, those focusing on liver health should stack with NutraBio Milk Thistle, and read our similarly in-depth article to understand why.
Dosage Suggestions
NutraBio suggests that you take one capsule daily, or as directed by your physician.
In more critical conditions, if you want to mimic the landmark study discussed above,[20] you'd need to take 250 milligrams (one capsule) three times daily. However, for general hepatic maintenance, you can likely dose less in order to make the bottle last longer.
Third party lab-testing is key with TUDCA

New to NutraBio? Read The NutraBio Story!
Every time we write about TUDCA, we learn more about this fascinating compound, and it turns out that the scientific community is too - new research is always coming out on this ingredient!
Since 2017, NutraBio has published third-party lab tests on all of their supplement lots, but they're emphasizing their lab tests even more because TUDCA is difficult to source and there have been scams in this category. That's beyond unfortunate, because this supplement is too mission-critical to buy anything that hasn't been tested off-site forwards and back.
This is even more important than our emphasis on lab testing with NutraBio Plant Protein, since some plant proteins notoriously have far too many heavy metals inside. The point is, when it's critically important to know what you're putting in your body (which is always), NutraBio is the brand you can trust.
NutraBio Tudca – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.






Comments and Discussion (Powered by the PricePlow Forum)