While updating our AMP Citrate / DMBA article to include a new research study, we noticed one glaring issue from the study:
You can see this below in a screenshot from the study[1]:
Only in the supplement industry do you fail at breaking the law because someone else was scamming you
This is based upon two different testing labs, NSF (NSF International in Ann Arbor, MI in the USA) and RIVM (Netherland's National Institute for Public Health and the Environment), which used different eequipment that had different sensitivities.
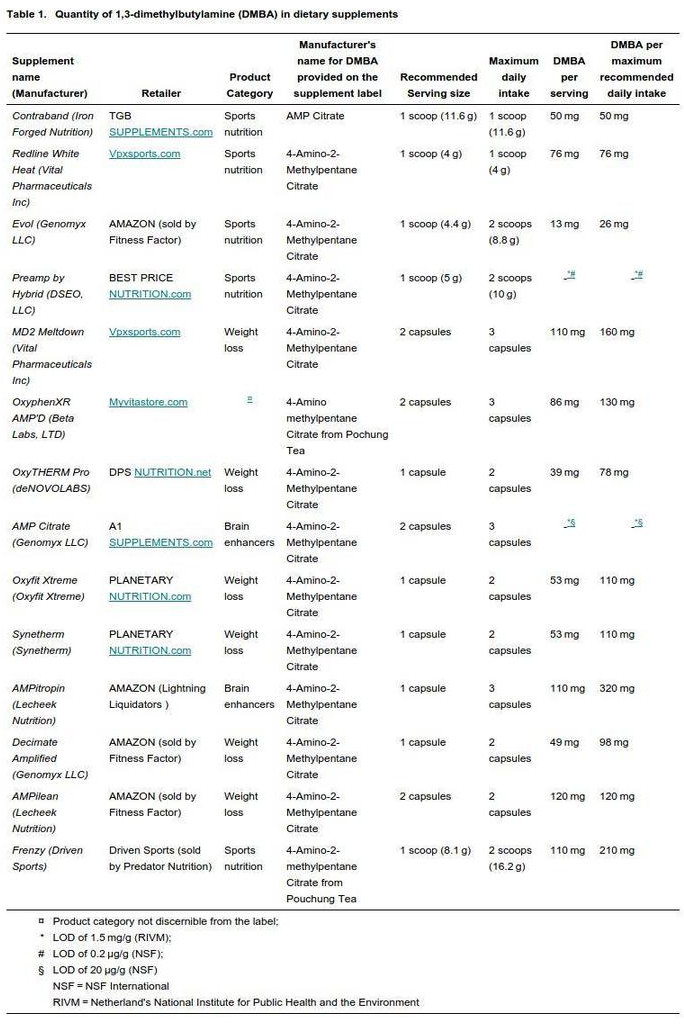
From the study's table above, the Genomyx AMP Citrate has a data point of -*# under the DMBA per serving field.[1]
What the legend means
- The - means that zero / none was found.
- The * means that the LOD, or limit of detection, was 1.5 mg/g (RIVM).
- The # means that the limit of detection for NSF was an impressive 0.2µg/gram.
Needless to say, we're confident that there's no AMP Citrate in this batch of Genomyx's AMP Citrate.
Update: We have asked Dr. Cohen what the batch / lot number(s) were on the bottles they tested. He never responded.
What if the tests for DMBA weren't good at finding AMP Citrate?
This is one question that's been asked - are we dealing with the right test here? Maybe their DMBA test isn't good at at finding AMP Citrate?
It should be noted that for the sake of testing, DMBA is the same thing as AMP Citrate, or 4-Amino-2-Methylpentane Citrate - they are just in different forms, but should have the same spectral peaks.
Besides, the two tests performed in the study found other products that contained AMP Citrate, too, which invalidates that argument. The ingredient is simply not in the products they tested.
So, what's in there?
This leads us to ask Genomyx (and their raw materials suppliers)... What is in this stuff?!
Because clearly, it's got some nice stimulant effects. "Other popular stimulant"-like, in fact... just as we'd expect from an AMP Citrate product!
Pieter Cohen, the Harvard Medical School assistant professor who led the study, did not have enough data to determine what was in it. They would need larger sample sizes to read much into the other UHPLC peaks.
Our take
We will possibly never know, what's going on, but this stuff should be recalled ASAP, or at least be thoroughly tested so consumers can be aware of what they're getting.
This is why companies must test their raw ingredients from manufacturers!
It's likely a bait-and-switch was done here, and while it may not have been with Genomyx's knowledge, it was done nonetheless. If you're holding a bottle of Genomyx AMP Citrate, there's no telling what you're really holding.
Only in this industry...
An industry insider source who wishes to remain unidentified stated,
"Only in the supplement industry do you fail at breaking the law because someone else was scamming you"
On a more serious note, he clairified,
"It should come as no surprise to supplement users that companies which are willing to operate in the grey area of ambiguous ingredient legality are also willing to skirt regulations requiring that they accurately label their products and have them batch tested for quality assurance"
Evol is low in DMBA too
Meanwhile, the other Genomyx product, Evol, has a paltry 13mg DMBA, so it looks like similar ingredient shuffling has occurred.
Note: The other supplement that contained no DMBA, but has it listed on the label as 4-Amino-2-Methylpentane Citrate, is Hybrid's Preamp pre workout supplement.
Expect recalls?
Anyway, we expect this one to be pulled / recalled, so if you like to live dangerously and eat unknown stimulants, check out the price comparisons below... and be careful!



Comments and Discussion (Powered by the PricePlow Forum)