2022 has been an awesome year for Anabolic Warfare. Their Project Muscle series has made some serious waves in the supplement scene, with nearly a dozen premium formulas designed to help consumers bulk, cut, and shred to their heart's content.

The next wave of warfare is here from Austin, TX based Anabolic Warfare -- Project Muscle! Inside we introduce the eleven incredibly unique supplements, and today we'll focus on the hormonally-minded Project Cuts weight loss supplement.
One of Anabolic Warfare's major claims to fame this year lies in their pioneering use of phytoecdysteroid newcomer turkesterone, which made an appearance in Project Hulk, Project Gains, and Project Bulk.
We're also pretty excited to see the classic muscle-synthesis-signaling ingredient, arachidonic acid (AA), make an appearance in Project Jacked, which we hope is the beginning of an AA renaissance.
Project Cuts: Weight Loss Through Hormone Balance
Although ingredients like turkesterone and AA are awesome anabolic agents, Project Muscle is, contrary to what you might expect from the name, not just about putting on muscle.
Earlier in the year we covered Project Shred, which is designed to help users achieve a lean, shredded look through the use of anti-estrogen and diuretic ingredients, both of which help decrease water retention, thus potentially improving the aesthetic of the user's physique.
Estrogen-Focused Approach Reappears in Project Cuts
With Project Cuts, another entry in the awesome Project Muscle line, we get a really unique and innovative approach to a weight loss supplement.
The standard approach to formulating a fat burner is to emphasize stimulants and non-shivering thermogenesis to increase basal metabolic rate above baseline, thus accelerating the process of weight loss.

Anabolic Warfare Project Cuts helps accomplish weight loss through hormonal balance, not just stimulation!
Project Cuts doesn't do this. While it contains mangiferin, which does have some stimulant properties, the overwhelming emphasis of this formula is on hormonal balance.
We have a ton of aromatase inhibitors which, as we were saying about Project Shred, is going to help improve your appearance by minimizing estrogen production and hence, unnecessary water retention.
But the anti-aromatase activity does more than that: Since aromatase converts testosterone into estrogen,[1] inhibiting aromatase is going to help increase your testosterone levels[2] and offset any potential drop in testosterone production caused by caloric restriction.[3]
Since high testosterone production supports healthy body composition and energy levels, this is ultimately going make your cut way easier to deal with.[4]
As we'll see in our detailed discussion of the ingredients, estrogen production occurs mostly in subcutaneous fat. So if you've got a lot of weight to lose, aromatase activity converting testosterone to estrogen, which predisposes you to weight gain and further exacerbates the aromatase problem, can set up a nasty energy-draining positive feedback loop.
What's really smart about Project Cuts is it's designed to help the user break that feedback loop.
But testosterone and estrogen aren't the only hormones affected by Project Cuts' ingredients: We also have some intriguing thyroid hormone support, which is arguably just as important as aromatase inhibition when restricting calories.
We'll get into that in a minute, but first, let's check Anabolic Warfare news and deals from PricePlow:
Anabolic Warfare Project Cuts – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.
Ingredients
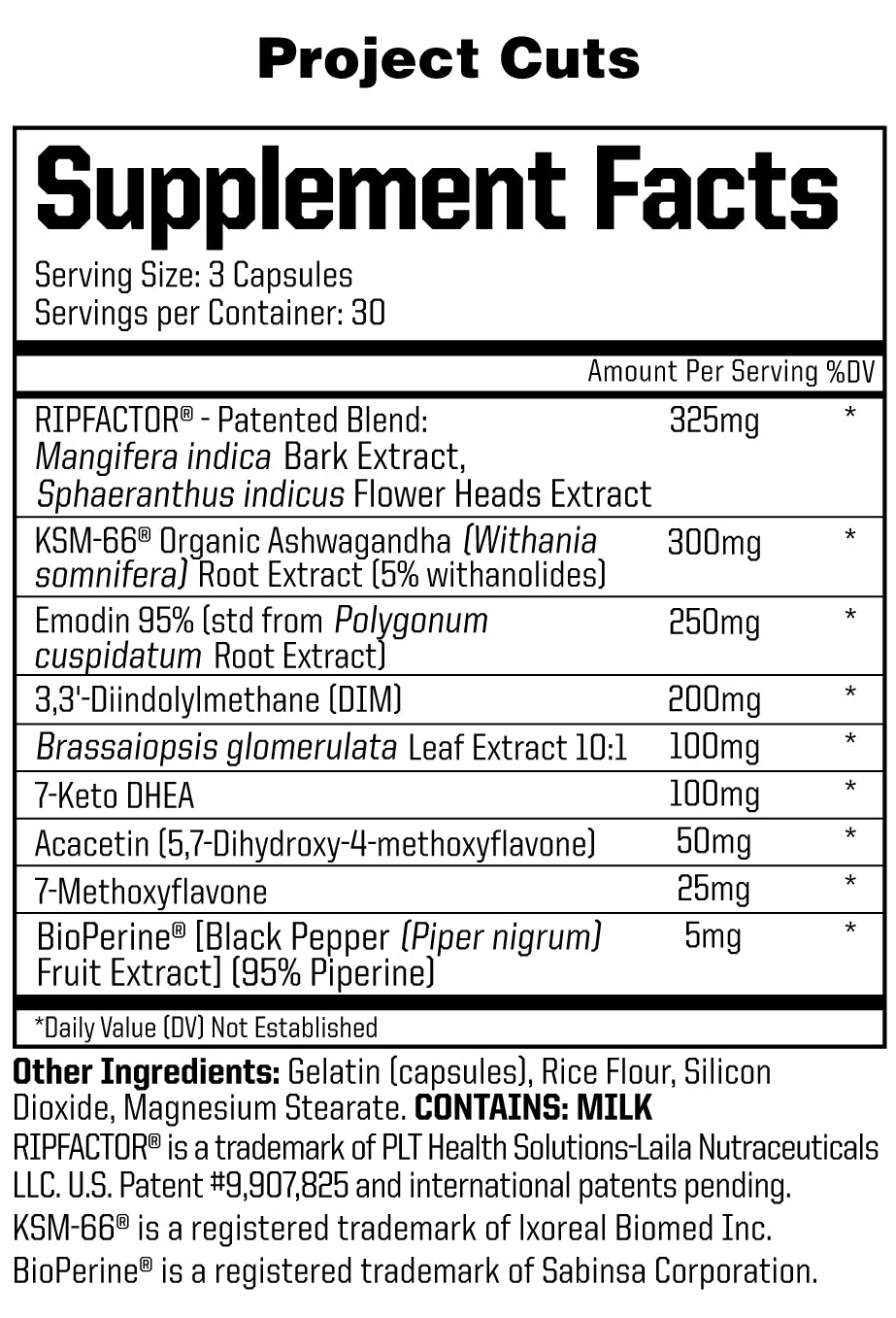
In a single, 3-capsule serving of Project Cuts from Anabolic Warfare, you get the following:
-
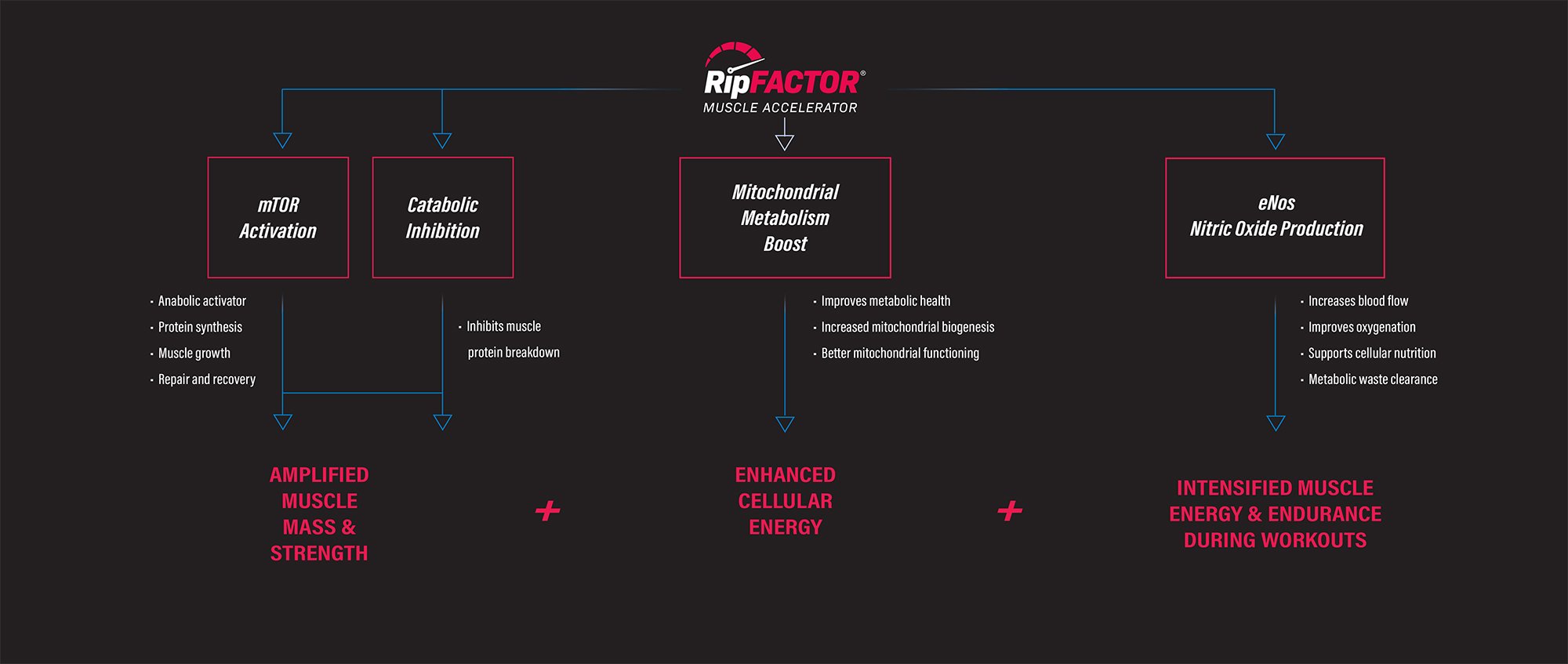
RIPFACTOR (Mangifera indica & Sphaeranthus indicus) – 325 mg
RIPFACTOR is a trademarked blend of extracts from Mangifera indica, which is referred to colloquially as the mango fruit and Sphaeranthus indicus, a flowering plant whose common name is East Indian globe thistle.
Both of these plants have a long history of use in the system of Ayurvedic medicine,[5,6] which holds them to possess a wide range of medicinal properties that could broadly be described as energizing.
First let's talk about the Mangifera indica extract, which is standardized for a bioactive xanthone called mangiferin.
Astute students of supplement science will have noted the similarity between the words xanthone and xanthine, and if your intuition told you that caffeine-like effects might be at play here, congratulations: The xanthone mangiferin is indeed similar in its action to the xanthine caffeine.
Like caffeine, mangiferin evolved as a pesticide produced endogenously by the mango tree in order to defend itself against predation by insects. Also like caffeine, mangiferin has stimulant effects on mammalian central nervous systems[7] and nootropic properties.
For instance, supplementation with mangiferin has been demonstrated to facilitate a process called long-term potentiation,[8] which occurs in the human hippocampus and consolidates short-term memories into long-term ones. That's a fancy way of saying that mangiferin improves learning.
Mangiferin actually synergizes with caffeine, too -- the effectiveness of these compounds given in combination exceeds that of either compound alone.[8] We don't have any caffeine present in Project Cuts from Anabolic Warfare, but given the ubiquity of caffeine use, we would consider this synergy to be a potential benefit for the vast majority of consumers.
Where Sphaeranthus indicus enters the equation is its high content of quercetin, an antioxidant that also synergizes with mangiferin. The combination of quercetin and mangiferin has been shown in the research literature to significantly improve several dimensions of athletic performance, including really important ones like VO2 max, peak power, and oxygen uptake.[9,10]
The effectiveness of quercetin and mangiferin together is both short-term and long-term[9,10] – you can get benefits immediately after taking them, but the benefits compound, to an extent, the longer you take them.
One study from 2020 showed that mangiferin and quercetin decreased muscle damage and soreness after exercise, and significantly sped up recovery.[11]
-
KSM-66 Organic Ashwagandha (Withania somnifera) Root Extract (5% withanolides) – 300 mg
Ashwagandha is a great adaptogenic herb, meaning that it helps your body deal with stress. That's really important when on a cut, since restricting calories can reduce thyroid hormone and testosterone production, while increasing stress hormones like cortisol.
In one 2012 study, 64 adults with a history of chronic stress were able to achieve significant reductions in their stress levels by taking the same KSM-66 brand extract used in Project Cuts.[12] Importantly, this study measured stress both objectively with blood tests for stress hormones, and subjectively with questionnaires designed to measure the emotional state of the respondent.
So KSM-66 wasn't just protecting the study volunteers' physical health – they felt better emotionally too, as confirmed by other studies.[13]
Cortisol and testosterone are antagonistic toward each other – they have broadly opposite actions in the human body, and generally speaking, anything that raises cortisol is going to lower testosterone, and vice-versa. So with KSM-66's ability to reduce cortisol production in mind, it's probably not surprising that supplementation with the compound has also been shown to raise testosterone levels. In one study, the effect size ranged from a 14% to 40% boost in testosterone, which is not just significant, but impressive.[14]
In another, similar study, ashwagandha was found to boost testosterone in male subjects by 10% to 22%[15] – not as big of a boost, but then, this study used a generic extract instead of KSM-66, which could be a factor in the less impressive result.
Importantly, this testosterone-boosting effect is not only seen in hypogonadal or infertile men. It also occurs in active, young, healthy men – a population whose testosterone levels are generally close to optimal before supplementation.[15]
Testosterone levels are a significant factor in athletic performance, so naturally, ashwagandha has also been shown to increase both power output and VO2 max[16] in men who take it. One study found that ashwagandha supplementation was able to increase participants' leg press and bench press one-rep max weight.[17]
-
Emodin 95% (std from Polygonum cuspidatum Root Extract) – 250 mg
Emodin is an anthraquinone that can be isolated from plants like rhubarb, buckthorn, and Japanese knotweed.
To understand what emodin does and how it works, you first need to understand the cross talk between the two basic types of human adipose tissue: white adipose tissue (WAT) and brown adipose tissue (BAT).
WAT is the form of fat your body makes to store long-term energy reserves, the kind it will draw on in the event of severe famine, where your caloric intake is dangerously low. This is a good thing in the sense that it's a crucial survival mechanism – human beings evolved to put on WAT for a very good reason, which was that in our evolutionary past, food was often scarce. Without WAT, it's unlikely that your ancestors could have survived long enough to bring you into existence.
BAT, on the other hand, is a metabolically active form of fat. Under certain circumstances, WAT becomes BAT through mitochondrial biogenesis, and in fact the mitochondria themselves are what gives BAT a dark brown appearance compared to WAT when viewed under a microscope.
Although there are several triggers for WAT-to-BAT conversion, this process is usually initiated by cold exposure,[18] which makes sense once you learn that BAT's main function is non-shivering thermogenesis, a process in which your body converts calories (in the form of carbs or fatty acids) into heat.
Thermogenesis is a mechanism for raising or maintaining your core temperature.[19] The name is a little confusing because it seems to imply that non-shivering thermogenesis doesn't happen when you're cold. But all the "non-shivering" part really means is that the calories are being burned by mitochondrial uncoupling, rather than muscular activity (shivering).
Basically, the more BAT you have compared to WAT, the more calories you're going to burn at rest.[20,21] In other words, BAT speeds up your metabolism, making it easier to lose weight and keep it off.
BAT doesn't just burn fat though – it can also use glucose for thermogenesis, meaning that having more BAT can improve your blood sugar and triglyceride profile even in the absence of weight loss.[22]
OK – with all that out of the way, how does emodin actually work?
Emodin drives the conversion of WAT to BAT![23]
It does this by signaling adenosine monophosphate-activated protein kinase (AMPK),[24] an enzyme that's responsible for governing whole-body energy balance, and plays a crucial role in "browning" WAT.[25]
Even though this extract is standardized for 95% emodin, it seems worth mentioning that Polygonum cuspidatum, colloquially known as Japanese knotweed, contains other compounds that cause WAT-to-BAT conversion. Resveratrol is just one example.[26]
-
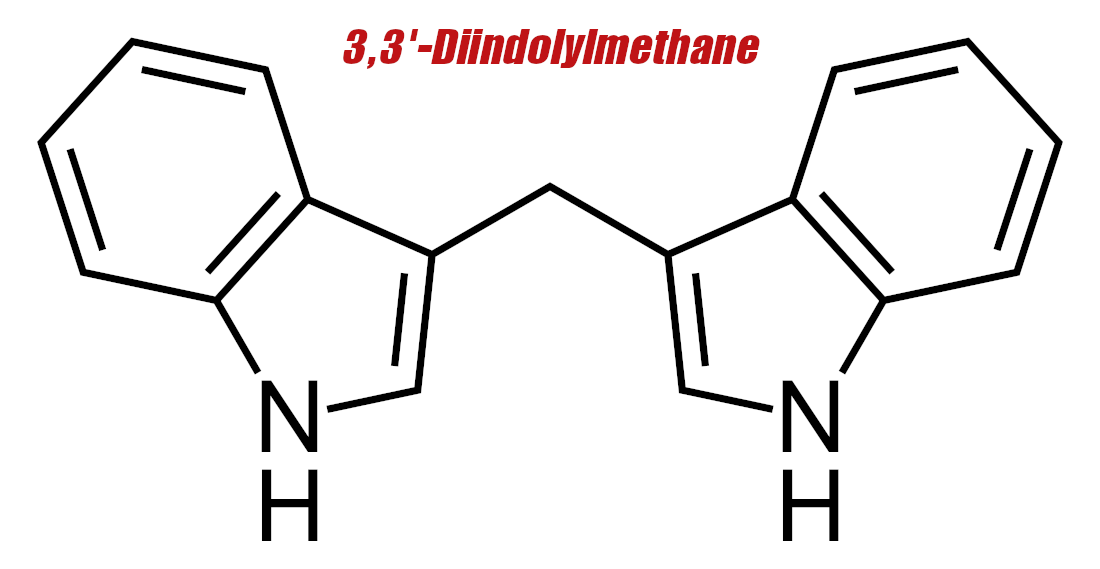
3,3'-Diindolylmethane (DIM) – 200 mg
DIM is a metabolite of 3-indole-carbinol, which in turn is a metabolite of glucobrassicin, a glucosinolate that occurs naturally in cruciferous vegetables. In general, glucosinolates are what give plants like mustard, cabbage, and horseradish their pungent flavor. All of these plants belong to the Brassica family, of which glucosinolates are a key characteristic.
DIM can improve human hormonal health by inhibiting aromatase, an enzyme that converts testosterone into estrogen. Although we (meaning women and men) do need a little estrogen for optimal health,[27] aromatase is overexpressed in many people due to our modern environment and lifestyle,[28] which unfortunately exposes us to many estrogen-like endocrine disrupting compounds called xenoestrogens.[29,30]
Increased aromatase activity can lead to a condition called estrogen dominance, where your estrogen to progesterone ratio is too high.[31]
Estrogen dominance is pretty bad news for anyone – male or female – who happens to be afflicted by it. The onset of estrogen dominance is associated with many diseases, including some forms of cancer.[32]
The issues with estrogen dominance in both men and women
But estrogen dominance itself can cause plenty of other problems too.
In women, the list of symptoms is huge, and runs the gamut of maladies:[33]
- Bloating
- Decreased sex drive
- Irregular periods
- Mood swings
- Headaches
- Anxiety attacks
- Weight gain
- Hair loss
- Cold hands and feet
- Insomnia
- Fatigue
- Memory problems
In men, estrogen dominance tends to manifest as sexual dysfunction – loss of desire and/or difficulty achieving and maintaining an erection. In extreme cases, estrogen dominance may cause men to develop breast tissue.[33]
The arrow goes both ways, too: aromatase overexpression can lead to disease, but disease can also lead to aromatase overexpression. One example of this would be obesity, as adipose tissue (also known as body fat) is the site of aromatase production.[34,35]
Interesting note – we tend to hear a lot about how visceral fat is the most damaging form of fat, and in many ways it is – but when it comes to estrogen production, subcutaneous fat is actually worse.[36]
So if you want to optimize your hormonal health, you really do need to get lean. It's not enough to just decrease the amount of visceral fat in your body.
DIM inhibits aromatase expression
So now we know about the dangers of aromatase overexpression and estrogen overproduction. How does this relate to DIM?
DIM acts as an aromatase inhibitor, helping us turn off aromatase and avoid the many pitfalls associated with having too much estrogen.[37]
Moreover, DIM has a high affinity for the aryl hydrocarbon receptor (AhR), which downregulates estrogen receptors.[32,38,39] Increased AhR activity ultimately means that the estrogen circulating in your blood will have less of an effect on your health since it has fewer cellular receptors to bind to.[40]
DIM is helping decrease your overall estrogenic burden with a one-two punch: by both decreasing estrogen production via the downregulation of aromatase, and blocking the estrogen you do produce at the receptors.
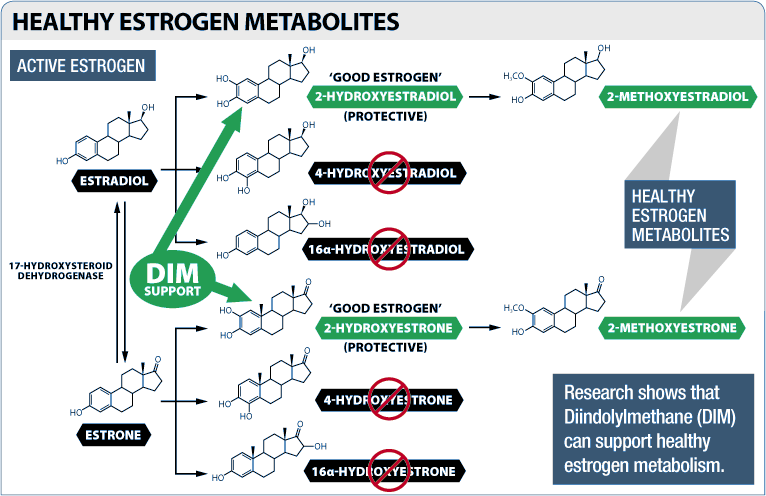
DIM also improves your body's ratio of good and bad estrogen metabolites
Besides reducing the overall activity of estrogen, DIM can also encourage the production of good estrogens over bad estrogens.
The three main forms of estrogen produced by the human body are estrone, estradiol, and estriol. Estradiol (E2) is the strongest estrogen of the three, meaning that it stimulates estrogen receptors the most.[41] Generally speaking, E2 is the form of estrogen that we're trying to minimize (but don't go overboard with this because it is very possible to become E2 deficient).
These three forms of estrogen are broken down by various metabolic processes into the estrogen metabolites, a category that includes estradiol-2-hydroxylase (EH), 4-Hydroxyestradiol (4-OH-E2), and 16-alpha-hydroxyestrone (16OH-E1).[41]
The scientific evidence suggests that 2-hydroxylated estrogen is "good" estrogen, and can help improve many aspects of human health.[42,43] Among other things, EH does not encourage the uncontrolled cellular division that estrogens like estradiol/E2 can.[44] This means that, in theory, having higher EH levels should not increase your risk of cancer.
On the other hand, 16-hydroxylated estrogen is regarded as being significantly carcinogenic and has a much higher affinity for the estrogen receptor than EH, meaning that it makes a more significant contribution to your body's overall estrogenic burden.[44,45]
The 4-hydroxylated form of estrogen is so carcinogenic that one peer-reviewed study proposes it be used as a biomarker for breast cancer.[46,47]
Primarily for these reasons, researchers are beginning to accept the ratio of 2-hydroxylated estrogen to 16-hydroxylated and 4-hydroxylated estrogens in a person's body as a measure of their overall hormonal health.[44]
Having a higher proportion of 2-hydroxylated estrogen is associated with greater lean mass and lower levels of body fat.[48]
As it turns out, DIM can help improve your body's estrogen metabolite ratio. It has been shown to increase your levels of the 2-hydroxylated forms while decreasing your levels of the 4-hydroxylated and 16-hydroxylated forms.[49-52]
-
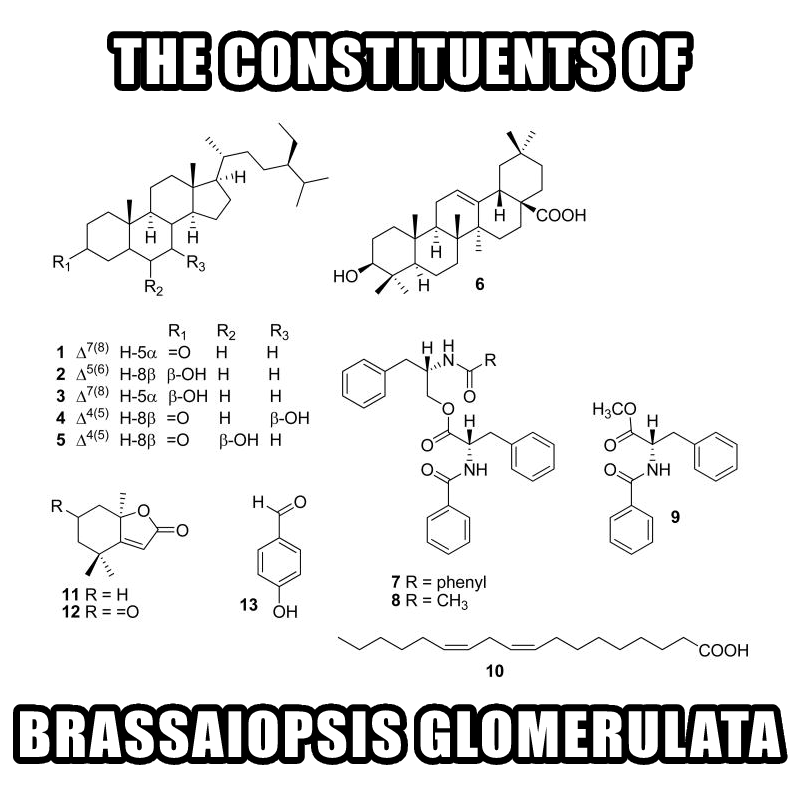
Brassaiopsis glomerulata Leaf Extract 10:1 – 100 mg
The constituents of Brassaiopsis Glomerulata. We're most interested in #9, N-benzoyl-L-phenylalanine and #12, dehydrololiolide.[53]
The tree Brassaiopsis glomerulata is native to Vietnam, and is rich in aromatase-inhibiting compounds. Among these are dehydrololiolide and N-benzoyl-L-phenylalanine, the bioactive constituents of Brassaiopsis extracts.[53]
The latter compound, often referred to in the research literature as N-benzoyl-L-phenylalanine methyl ester, actually has a history of synthetic production but was not observed in nature until recently.[53]
This is a reversal of the usual dynamic in the discovery of new drugs, where a compound is first observed in nature and then created synthetically to reduce cost and improve access.
The fact that scientists independently came up with N-benzoyl-L-phenylalanine methyl ester for the purposes of aromatase inhibition is a testament to its potency – it's basically a designer drug.
-
7-Keto DHEA – 100 mg
7-Keto DHEA is a metabolite of dehydroepiandrosterone (DHEA). It has been shown to increase metabolic rate by reducing cortisol and increasing thyroid hormone production.[54-56] We last covered it in Anabolic Warfare Project Jacked, where it's used in a pro-inflammatory muscle builder to maintain leanness.
Arachidonic Acid makes a return in Anabolic Warfare Project Jacked. This is the last time we covered 7-Keto DHEA, where it's used to maintain leanness while inflaming the muscle.
Your basal metabolic rate, the amount of energy your body burns at rest in a day, is determined largely by your thyroid function.[57] Adequate levels of the two thyroid hormones – triiodothyronine (T3) and tetraiodothyronine (T4) – are absolutely essential for properly metabolizing both glucose and fatty acids.[57]
Obviously it's important to have a fast metabolism, so optimal thyroid function is important under the best of circumstances. But when you're restricting calories to lose weight, it becomes even more important because low-calorie diets are associated in the research literature with reduced thyroid function. According to one study, a 1,200-calorie diet can reduce your T3 levels by over 50%, which is a huge effect size.[58]
The mechanism of action behind 7-keto DHEA's pro-metabolic effect seems to be its ability to increase serum levels of triiodothyronine (T3), your body's active thyroid hormone.[54]
A randomized, double-blind, placebo-controlled study published in 2000 found that 7-keto DHEA supplementation was actually able to reverse the decline in thyroid function and hence, basal metabolic rate that typically accompanies caloric restriction.[54]
This is significant, as the typical metabolic drop is what often leads to weight loss plateaus for people who are dieting for extended periods of time.
-
Acacetin (5,7-Dihydroxy-4-methoxyflavone) – 50 mg
Here we have another incredibly potent aromatase inhibitor in the form of acacetin.
According to one 2008 study, which set out to compare the ability of 24 different compounds to inhibit aromatase activity, acacetin was one of the two most potent.[59]
Acacetin has been shown to increase libido and restore sexual function in sexually exhausted male rats.[60]
-
7-methoxyflavone – 25 mg
The methoxyflavones are known in general for their aromatase-inhibiting properties.[61] Acacetin, which we discussed above, is one example of a methoxyflavone, but it's not the only one: there's also 7-methoxyflavone, another powerful aromatase inhibitor.[61]
By now, you should understand why aromatase inhibitors are good and how they work, but if you need a refresher, scroll back up to the DIM section above.
-
BioPerine (Black Pepper (Piper nigrum) Fruit Extract) (95% Piperine) – 5 mg
BioPerine is a black pepper extract that's standardized for the alkaloid piperine, and is most often used as a bioavailability enhancer. By inhibiting the action of certain stomach enzymes, BioPerine can spare nutrients from first pass digestion, allowing them to transit through the stomach and reach the intestines where they're absorbed into the bloodstream.[62]
This matters because unless a supplement gets into your blood, it's probably not going to have any significant effect on your health. Obvious exceptions to this rule would be certain kinds of gut health supplements that are designed to be broken down in your digestive tract.
We like seeing bioavailability enhancers included in formulas because they help to maximize the value that you, the consumer, get in return for the dollars you spend on supplements.
So BioPerine increases the effectiveness of other ingredients, but it also, in the context of a fat loss supplement, has some independent benefits that can help you achieve your goals.
For instance, piperine has been shown to upregulate glucose transporter 4 (GLUT4), a transporter protein that helps move glucose out of your blood and into your body's cells where it can be burned as energy instead of driving up your blood glucose level.[63]
BioPerine has also been observed to help improve insulin sensitivity and decrease fatty liver in mice,[64] partly because of its effect on glucose metabolism but also because of its antioxidant properties.[65]
Conclusion
One of the things we've noticed about Anabolic Warfare's approach to supplement formulation is that, instead of throwing the proverbial kitchen sink at the problem, they identify one major factor and go all-out with synergistic ingredients to deal with it.
In this case, the one major factor is aromatase expression, which we think is a great target for a weight loss or fat burning supplement.
Remember, the Anabolic Warfare Project Muscle Series formulas are designed to be stacked with each other, so if there's some other factor in body composition that you also want to address, be sure to check their catalogue for something that would complement Project Cuts.
Anabolic Warfare Project Cuts – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.











Comments and Discussion (Powered by the PricePlow Forum)