We're excited to unveil to you the weight loss supplement everyone's been waiting for: A diet supplement featuring a new natural ingredient that significantly boosts GLP-1 and suppresses appetite!
In today's fast-evolving weight management space, finding a natural alternative to the popular GLP-1 agonists is on everyone's radar. And we believe Modere has truly figured it out.
Curb by Modere: A GLP-1 Boosting Weight Loss Supplement That Increases Satiety

Modere CURB introduces Eriomin-powered GlycoLemon for natural appetite suppression and enhanced gut health, setting a new standard in stimulant-free weight loss support!
Enter Modere's Curb, a stimulant-free, flavored powder that taps into the body's natural mechanisms to help suppress appetite. With the power to support GLP-1 (Glucagon-like Peptide-1) – a hormone responsible for regulating appetite – Curb offers a safe, non-prescription way to promote satiety.
Its secret weapon? GlycoLemon, a new and exciting ingredient featuring Eriomin, which has been shown in clinical studies to significantly reduce hyperglycemia, enhance GLP-1 levels, decrease systemic inflammation, and improve gut health.
New Citrus Bioflavonoids Meet Fiber for the Ultimate Natural 1-2 Punch
Curb is developed with a comprehensive blend of fibers and botanicals to support weight loss, enhance gastrointestinal health, and promote overall wellness. Its key ingredients, including konjac root-derived glucomannan and resistant potato starch, expand in the stomach to increase fullness while supporting the growth of beneficial gut bacteria like Akkermansia – known for its association with lean body composition.
The unique formula also promotes blood glucose management and may reduce collagen breakdown, tying into both weight loss and beauty-from-within benefits.
The next Jen Anderson X Nate Frazier Masterpiece

Nate Frazier & Ken Huntly of GNC join the PricePlow Podcast for Episode #098 to discuss the Kaged partnership and GNC's strategy in 2023
This isn't just any supplement – Curb was created by Jen Anderson, a chemist with 16 years of experience at Albion, working alongside Nate Frazier, the former COO of GNC and CEO of Modere, who joined us on PricePlow Podcast Episode #098. With such a powerhouse duo behind it, Curb represents a game-changing solution in the weight loss market.
This is Modere's biggest launch since their cornerstone supplement, Liquid BioCell Collagen. That product's success has been massive -- and we believe this will be even greater.
Ready to curb those cravings and elevate your weight loss journey? Check out PricePlow's deals and sign up for our Modere news alerts to stay in the loop, then let's get into the scientific details:
Modere Curb – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.
This area is reserved for Team PricePlow's upcoming videos.
Subscribe to our channel and sign up for notifications so you catch it when it goes live!
Curb’s Primary Mechanism of Action – Increasing GLP-1 Production
By now, nearly everyone has heard of Ozempic (semaglutide), an injectable peptide that stimulates the receptor for a hormone called glucagon-like peptide-1 (GLP-1).
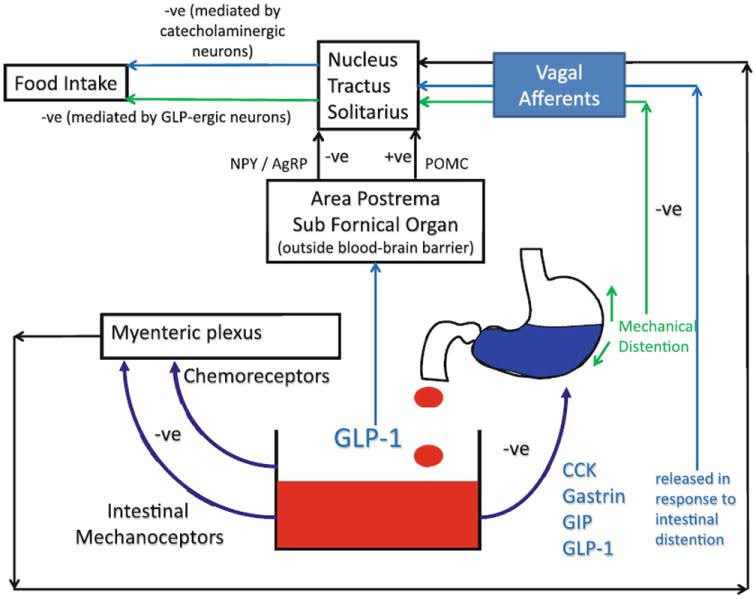
GLP-1 modulates appetite through two primary mechanisms:
- First, it upregulates key gastrointestinal hormones like CCK, gastrin, and GIP, which support digestion and send satiety signals to the brain.
- Second, GLP-1 promotes feelings of fullness through both hormonal and mechanical means. The aforementioned hormones not only induce a sensation of fullness, but also slow the rate of gastric emptying, keeping food in your stomach longer.[1] This effect is illustrated in the inset graph as "mechanical distention"[1] and is known as gastroparesis.[2,3]
Increased feelings of satiety can reduce spontaneous food intake over time, leading to improved insulin sensitivity and glycemic control in the long run. Additionally, GLP-1 agonism directly influences insulin secretion and response.
Notably, GLP-1 is a selective insulin secretagogue. More specifically, it's an incretin hormone that triggers insulin secretion only in response to food intake, as its effects are glucose-dependent and primarily activated when blood glucose levels are elevated. This targeted insulinogenic action helps maintain postprandial blood glucose levels within a normal range.[4]
Furthermore, upregulating GLP-1 decreases the secretion of glucagon, a hormone produced by pancreatic alpha cells that raises blood glucose levels by stimulating the conversion of liver glycogen to glucose. Type 2 diabetes is typically characterized by excessive glucagon secretion; thus, reducing it through GLP-1 agonism can help normalize blood glucose levels in diabetics.[5]
GLP-1 upregulation can also directly enhance insulin signaling. While the exact not yet fully understood, research indicates that GLP-1 promotes the translocation of glucose transporter type 4 (GLUT4) to the surface of muscle and fat cells, facilitating cellular glucose uptake from the bloodstream and consequently lowering blood glucose levels.[6]
In a related manner, GLP-1 agonists can combat lipotoxicity by promoting the breakdown of fatty acids.[7]
Collectively, these mechanisms contribute to significant improvement in mitochondrial function, particularly in individuals with diabetes.[8] As regular readers of the PricePlow Blog already know, mitochondrial health is critical for both mental and physical performance, as well as long-term health and wellness.
Can dietary supplement ingredients mimic these effects more safely?
It's no surprise that the dietary supplement industry is pursuing these effects.
However, Ozempic almost seems to work too well. The condition of gastroparesis,[2,3] where gastric emptying is delayed by the drug, causing the stomach to take too long to empty its contents,[9-12] may contribute to some severe side effects and concerns about future gastrointestinal issues and diseases. There's nothing quite like food rotting in one's stomach, especially when it consists of ultra-processed items from the Standard American Diet.
Can the supplement industry do better with natural ingredients? We believe so, and Modere is on a path to prove it with the novel ingredients in Curb:
Modere’s Curb Ingredients
The first two ingredients are included to enhance fullness and satiety, the most commonly-sought effect for users of drugs like semaglutide. Following that, we introduce a new trademarked GLP-1 booster that holds extraordinary promise.
In a single serving (6.3 gram packet) of Curb from Modere, you receive the following:
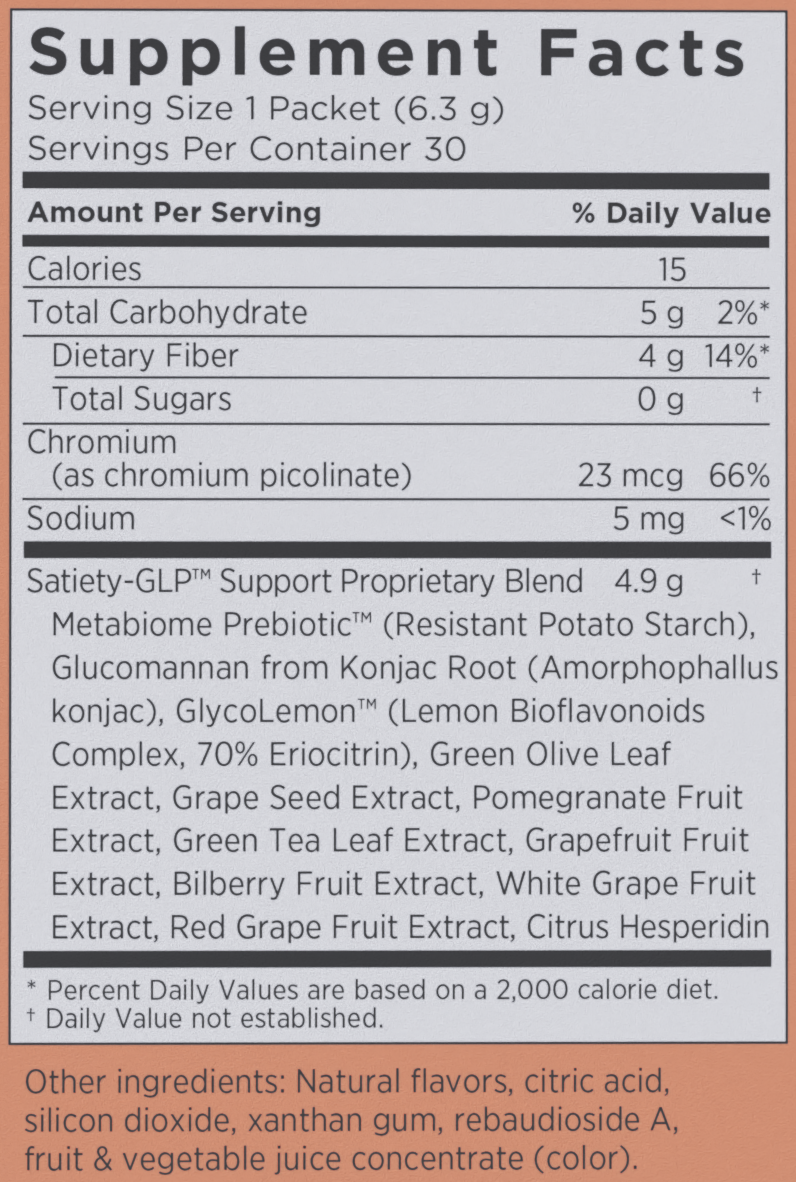
Modere's Curb may have a proprietary blend, but we know how much fiber is in here, and GlycoLemon is their trademarked blend that includes a clinically-verified dose of the potent GLP-1 agonist ingredient Eriomin, which is the focus of our article on this novel product.
-
Metabiome Prebiotic (Resistant Potato Starch as Solnul)
Resistant starch has been shown to upregulate the production of short-chain fatty acids (SCFAs),[13] especially butyrate, which activates enteroendocrine L-cells in the gastrointestinal tract, responsible for secreting GLP-1.[14] Although it's not on the label, this ingredient is sold as as Solnul in the dietary supplement market.
There's strong evidence that the bacterial fermentation of dietary fiber can increase GLP-1 levels and feelings of satiety.[15] However, resistant starch is particularly effective due to its impact on SCFAs.
Multiple studies show that resistant starch can acutely and significantly reduce food intake compared to placebo when overweight/obese individuals are provided ad libitum food over the course of 24 hours.[16,17] Interestingly, they didn't report more satiety -- they just ate less.
Increases Akkermansia levels
Akkermansia muciniphila is a beneficial bacterium that resides in the gut microbiota. It plays a crucial role in maintaining the integrity of the gut lining and promoting a healthy immune response. This microorganism is known for its ability to break down mucin (a component of the gut lining), contributing to gut barrier health and metabolic balance.
Studies have linked Akkermansia to improved gut health, reduced inflammation, better metabolic outcomes (such as improved insulin sensitivity), and potential weight management support. A healthy population of Akkermansia is often associated with reduced risk of metabolic disorders and enhanced gut barrier function.
Solnul has been clinically shown to significantly increase the abundance of Akkermansia in the gut.[18] In a study included in the Solnul research, consumption of resistant potato starch led to a noticeable increase in Akkermansia levels, improving gut health and promoting better digestion.
The increased population of Akkermansia suggests that Solnul may help enhance gut barrier integrity and metabolic functions, such as blood sugar regulation and weight management, through its prebiotic effects, which encourage the growth of beneficial gut bacteria.
So while this may not make one feel fuller (that's the job of the next ingredient), the net effect is what many dieters are really looking for: decreased energy intake.
-
Glucomannan from Konjac Root (Amorphophallas konjac)
Glucomannan is another type of dietary fiber, which seems particularly effective (compared to other types of fiber) for facilitating fat loss.[19] It can form a solid, low-energy gel when hydrated, and promotes a satiety effect and lower food intake when used in a meal.[20]
In one 2005 study, supplementation with glucomannan not only led to significant fat loss, but also significantly improved key biomarkers related to blood lipids and glycemic control.[21]
For example, other research has found that glucomannan can lower serum glycerides, in addition to total cholesterol and LDL cholesterol.[22]
A one-two punch with 4 grams of fiber
With a total of 4 grams of fiber coming from resistant potato starch and glucomannan -- 14% of the recommended daily value (which next to nobody gets without supplementation) -- Curb is off to a great start. Resistant starch seems best at reducing food intake and glucomannan seems best at promoting the feelings of fullness. We haven't even gotten into the other well-known digestive benefits and toxin-binding capabilities of dietary fiber, either. But it's time to get into the powerhouse GLP-1 ingredient, GlycoLemon / Eriomin:
-
GlycoLemon™ Lemon Bioflavonoids Complex – 70% Eriocitrin (Eriomin)
Curb's main GLP-1 booster is an exciting ingredient named GlycoLemon™, which is powered by Eriomin®. GlycoLemon is the innovative star in Curb, and much of its efficacy stems from Eriomin, a citrus-based ingredient that's been shown to significantly boost GLP-1 levels, as detailed in the research in this article.
While many citrus bioflavonoids are available on the market, Eriomin stands out due to its composition, which includes a constituent called eriocitrin that makes up 70% of its makeup.
So, how effectively can we improve metabolic health using this exciting new nutraceutical GLP-1 agonist Eriomin? The short answer is: a lot.
There are already several rigorous, high-powered randomized controlled trials examining Eriomin's benefits for insulin resistance, glycemic control, and inflammation – and the results are astounding. This supplement's effectiveness far exceeds what we typically expect from a nutraceutical.
When it comes to improving certain biomarkers, Eriomin can even rival pharmaceutical GLP-1 agonists. Don't just take our word for it – let's dive into the research:
Effectiveness of Eriomin in managing hyperglycemia and reversal of prediabetes condition (2019)
This double-blind, randomized, placebo-controlled study tested Eriomin's ability to improve hyperglycemia and other biomarkers associated with prediabetes. It was conducted with a sample size of 103 prediabetic patients with an average age of 49.[23]
Participants were randomized to receive one of four treatments for 12 weeks:
- A placebo
- 200mg/day of Eriomin
- 400mg/day of Eriomin
- 800mg/day of Eriomin
Throughout this period, the researchers monitored key biomarkers of metabolic and hormonal health.
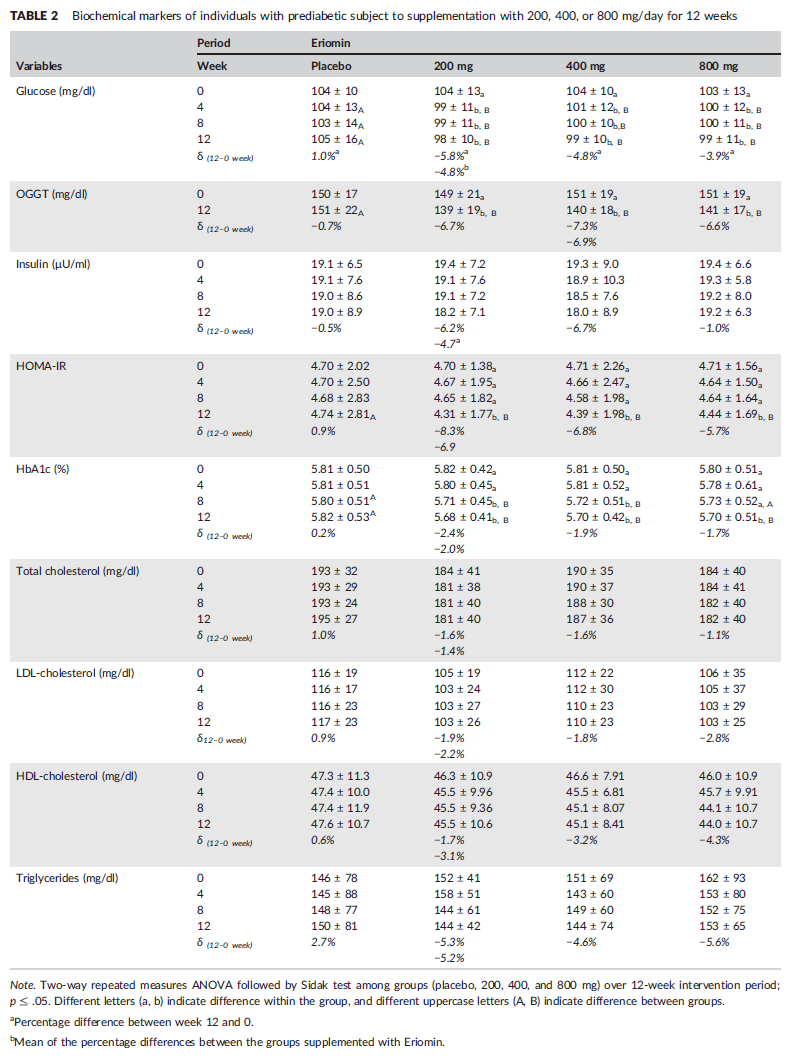
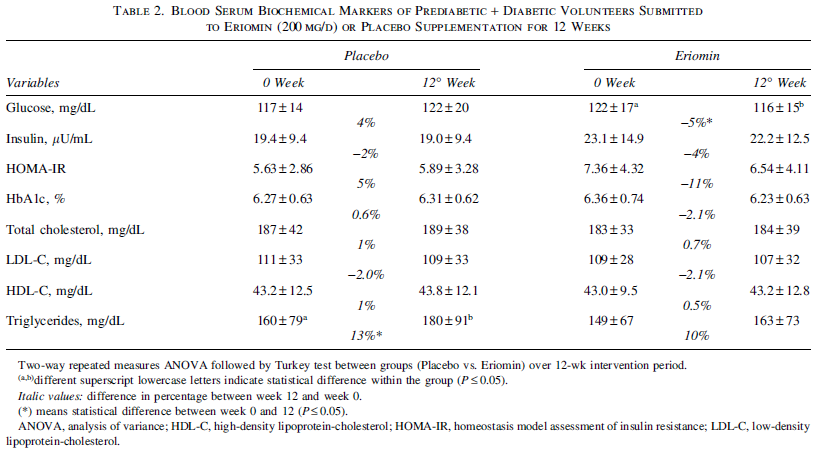
A summary table of results from the study.[23] We haven't highlighted anything specific here, because we'd end up highlighting everything – all the biomarkers listed were significantly improved by Eriomin supplementation.[23]
As shown in the inset table, all the biomarkers assessed in this study were significantly improved by Eriomin supplementation. The improvement in HbA1c particularly noteworthy, as this biomarker is the most commonly used for diagnosing diabetes and prediabetes.[24] A patient's HbA1c level measures the amount of glycated hemoglobin in the blood, serving as a measure of average blood glucose levels over the past three months.[25] It provides a more stable, long-term picture of glycemic control, indicating that statistically, the HbA1c number offers more signal and less noise than an instantaneous reading.
Here's what the study found:
Reduced HbA1c levels
First, and arguably most importantly, Eriomin decreased glycated hemoglobin (HbA1c) by about 2% across all Eriomin-supplemented groups. More specifically, the 200mg group saw a 2.4% reduction, while the 400mg and 800mg groups experienced reductions of 1.9% and 1.7%, respectively.[23]
The 200mg Eriomin group saw their HbA1c levels drop from 5.82% to 5.68%. While this may not seem like a huge difference in absolute terms, it resulted in 24% of the prediabetic patients enrolled in this study no longer being classified as prediabetic by the end of the study period.[24] In contrast, the placebo group experienced no reversals of prediabetes whatsoever.
Improvements in oral glucose tolerance testing
The subjects' scores on the oral glucose tolerance test (OGTT) also significantly improved. The OGTT, like HbA1c, is routinely used in the diagnosis of prediabetes and diabetes, and provides a direct evaluation of how the body handles glucose at the exact moment of administration.
Here's how it works: a patient is required to fast for 8 to 12 hours before the test. On testing day, they arrive at the clinic or lab for an initial blood draw, which measures fasting glucose. Afterward, they consume a drink containing 75 to 100 grams of glucose – usually 75 grams – so that the medical personnel on site can perform additional blood draws 1 and 2 hours after ingestion, providing a picture of how blood glucose levels change in response to the drink.[26]
The subjects' performance on the oral glucose tolerance test (OGTT) was significantly improved by all Eriomin groups, especially the 400mg/day dose.[23]
Looking at the data, you can see that the average blood glucose level 2 hours after the administration of the OGTT was about 150mg/dl at the beginning of the study and about 140mg/dl after 12 weeks of Eriomin supplementation. This is noteworthy because a blood glucose level of less than 140mg/dl is considered a normal result,[27] so Eriomin supplementation effectively reduced these patients' OGTT scores to right on the line between normal and pathological. In the 200mg/day Eriomin group, OGTT scores actually dropped below 140, meaning that Eriomin changed this group's results from pathological to normal.
Eriomin supplementation decreased fasting glucose in all groups from abnormally high (greater than 100mg/dl) to normal (70-100mg/dl).[23,28]
Reduction of fasting glucose levels
But the OGTT data is actually just the secondary outcome for glycemic control – the primary outcome defined by the study was fasting glucose, in which Eriomin was even more impressive. At the beginning of the study, all four groups of prediabetic subjects were well over the diagnostic threshold of 100mg/dL. After 12 weeks, all three Eriomin-supplemented groups saw their fasting glucose decrease to normal levels,[23] defined by the WHO as being between 70 and 100mg/dL.[28] This is an incredible result!
Given the observed improvements in fasting glucose, OGTT performance, and HbA1c levels, it's no surprise that homeostasis model assessment of insulin resistance (HOMA-IR) scores in all three groups dropped by 8% (200mg/day), 7% (400mg/day), and 6% (800mg/day).[23]
Nor is it surprising that other secondary outcomes improved – interleukin-6 (IL-6) and C-reactive protein (CRP) levels, both important measures of inflammation, dropped by 12% and 13% respectively.[23] Tumor necrosis factor alpha, another inflammatory marker, also decreased by 11%.[23]
It's also worth mentioning that systolic blood pressure dropped by 7-8% in all dose groups.[23] Since cardiovascular morbidity is a significant component of diabetes-related complications, this is another factor to pay attention to.
GLP levels increased by 15%!
So how do we know that GLP-1 was the mechanism here? Simple: the study measured it, and found that serum GLP-1 levels increased by an average of 15% among the study participants.[23]
Something interesting to note about this study is the lack of dose response in the findings. As the researchers point out, the Eriomin appeared to take full effect at 200mg/day, and for many biomarkers this lower dose actually performed better than 400mg or 800mg per day.[23]
This is great news, because the smaller the maximal effective dose, the lower the risk of side effects and the better the tolerability of the supplement. Conversely, it's generally true that the higher the dose required, the greater the risk of side effects.
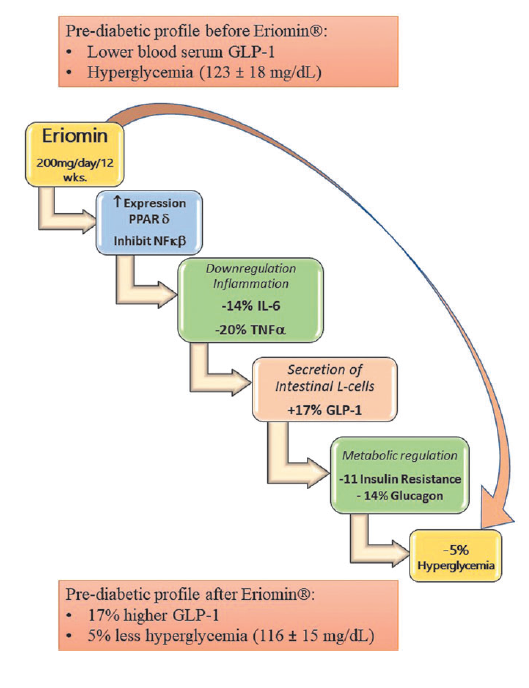
Reduces Hyperglycemia by Increasing Glucagon-Like Peptide 1 and Downregulates Systemic Inflammation (2022)
This study enrolled 45 participants aged 40 to 70, who had fasting glucose levels between 110 and 150mg/dl.[29] In other words, these patients weren't just pre-diabetic patients – some of them were full blown diabetic (the cutoff for diabetes is 125mg/dl).[28]
Unlike the last study we discussed, this was a crossover trial, meaning the subjects served as their own controls. They were randomized into two different groups, and each group received both treatment conditions. That is, the group that initially got Eriomin took it for 12 weeks, then stopped for a two-week washout period, before taking the placebo for 12 weeks – and vice-versa for the other group (placebo first, then Eriomin).[29]
The Eriomin dose used in this study was 200mg/day, a selection based on the previous clinical study, which, again, actually showed greater benefit at this dose than higher doses.[23]
as in the previous clinical study, Eriomin was found to significantly reduce fasting glucose, insulin, HOMA-IR, and Hb1Ac.[29]
Just like in the previous clinical study, Eriomin supplementation significantly improved key diabetes-related biomarkers. One really interesting thing to note is that in this population with more advanced diabetes, 200mg/day of Eriomin was actually much more helpful for reducing HOMA-IR – this study found an 11% reduction, compared to only 8% for the 200mg/day group in the previous study.[29]
This study also found similar increases in serum GLP-1 – 200mg/day Eriomin was found to increase it by 17%.[29]
Inflammatory biomarkers were also reduced – IL-6 by 14%, and TNF-a by 20%.[29]
Similar reductions in fasting glucose were noted – a 5% reduction in this study,[29] compared to a 5.8% reduction in the 200mg/day group from the previous study.[23] By comparison, the placebo condition caused no changes except a 13% increase in triglycerides.[29]
Although this study's findings are quite similar to those of the previous study, it does add a key insight: Eriomin works just as well to improve symptoms in patients with more advanced prediabetes, and even full blown diabetes.[29]
The researchers note that Eriomin could be a good alternative to conventional hypoglycemic agents, which some patients refuse because of their undesirable side effect profile. GLP-1 agonism is a mechanism of action typically not used by these drugs, and in the context of Eriomin, it appears to be a relatively safe mechanism, as no significant adverse effects were reported in this study or the other clinical study.
Improves gut dysbiosis in prediabetic patients (2023)
One big diabetes-related complication is gut dysbiosis, an imbalance in the gastrointestinal microbiome that's associated with the development and progression of diabetes. Under ordinary circumstances, healthy gut bacteria help the body maintain metabolic balance and glucose homeostasis. When bad bacteria predominate, the result is increased calorie absorption, fat gain, and insulin resistance – all important factors in the onset and progression of diabetes.[30]
Among other things, gut dysbiosis can cause intestinal permeability, a condition where toxic byproducts of cellular metabolism called lipopolysaccharides leak out of the gut and into the bloodstream, causing metabolic endotoxemia and chronic, systemic inflammation.[31,32]
In other words, gut health is absolutely crucial for maintaining metabolic health – and modulating the gut microbiota through dietary interventions, including supplementation with polyphenolic compounds like flavonoids, is one potential strategy to combat prediabetes.
So with that in mind, a 2023 study looked at how Eriomin could improve gut flora balance in prediabetic patients.
This double-blind, randomized, placebo-controlled trial enrolled 45 prediabetic participants aged to 69 years, with an average age of 50.[33] It once again used the 200mg/day dose of Eriomin, which had already been validated by the previous two clinical studies.
The average fasting glucose of these participants, which was 105mg/dL – significantly over the prediabetes cutoff of 100mg/dL.[33]
The study found that, once again, Eriomin significantly increased serum GLP-1 levels – they rose by 22% during the 12 week study period, and fasting blood glucose dropped by 6.5% in the Eriomin group, from 106mg/dL to 99mg/dL.[33]
None of that should surprise us – the previous two clinical studies both showed an increase in GLP-1 and improved glycemic control.
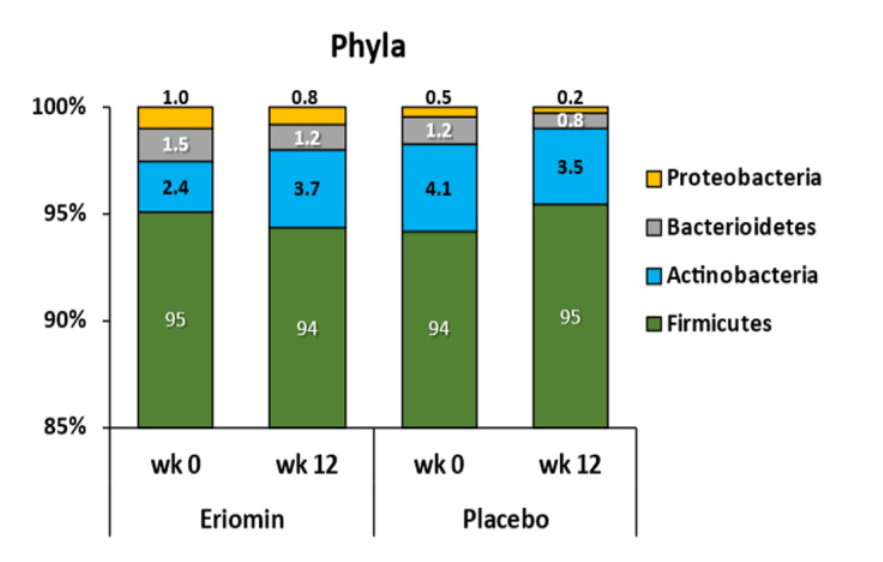
What's really interesting is how Eriomin supplementation altered species-specific composition of the subjects' gut microbiome.
Firmicutes bacteria are commonly linked to gut dysbiosis in prediabetic and obese individuals, which were overwhelmingly dominant in both groups' microbiomes at the start of the study (representing more than 90% of the subjects' total sampled bacteria). Both groups, unfortunately, experienced a growth in their Firmicutes populations during the 12-week study period, but the Eriomin group saw only a 25% increase, while the placebo group saw a 60% increase.[33]
Lachnospiraceae, which are also linked to glycemic dysregulation and obesity, increased by 11% in the placebo group but remained stable in the Eriomin group.[33]
Ruminococcaceae increased by 38% with Eriomin and decreased by 31% in the placebo group.[33] The reason this matters is that these bacteria are known to produce short-chain fatty acids (SCFAs), which increase insulin sensitivity and gastrointestinal function, while decreasing inflammation.[34]
Blautia bacteria, also linked to gut inflammation and metabolic endotoxemia, grew by 62% in the placebo group while remaining stable in the Eriomin group.[33]
There's so much more to Eriomin, including very promising preclinical data on its primary constituent, eriocitrin.[35-37]
-
Green Olive Leaf Extract
Olive leaf extract (OLE) contains bioactive constituents like oleuropein and hydroxytyrosol, which have documented anti-obesity effects.
A randomized, double-blind, placebo-controlled study from 2021 assigned obese women to one of two conditions: a calorie-restricted diet plus placebo, or a calorie-restricted diet plus OLE. The results were striking: the OLE+CR group lost an average of 4.1 kg, while the placebo group lost only 2.8 kg. This translated to a 1.6 point drop in BMI, compared to 1.1 for the placebo.[38]
Additionally, the OLE group's fasting blood glucose dropped by 4.3mg/dl on average, compared to 0.5 in the placebo group.[38] That's exactly the mechanism we want to see in a supplement like Curb.
-
Grape Seed Extract
Grape seed extract (GSE) has been shown to improve liver health, which is intimately related to metabolic health and often dysregulated in type 2 diabetes.[39]
In one study, scientists administered carbon tetrachloride, a powerful liver toxin, to rats. They found that rats who supplemented with GSE were largely protected from liver damage, thanks to GSE-derived proanthocyanidins' ability to downregulate enzymes that lead to hepatic lipotoxicity.[40]
In another study, proanthocyanidins upregulated liver concentrations of nicotinamide adenine dinucleotide (NAD+),[41] which profoundly benefits mitochondrial health and upregulates a pro-metabolic gene called sirtuin 1.[42]
Finally, GSE can increase the body's production of glutathione (GSH), a powerful endogenous antioxidant that can limit diabetes-related inflammation and oxidative stress.[43]
-
Pomegranate Fruit Extract
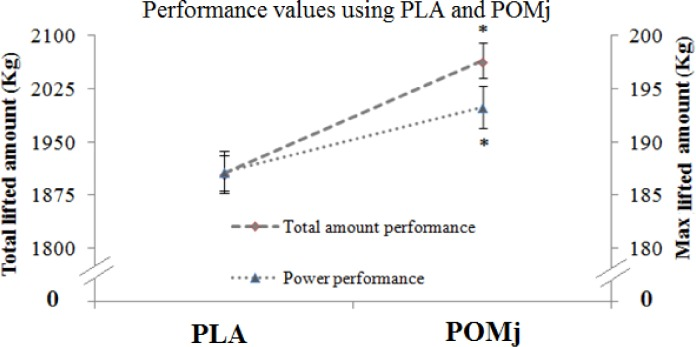
Pomegranate juice supplementation in trained weightlifters caused a significant increase in performance.[21]
Pomegranate (Punica granatum) is a rich source of powerful antioxidant, anti-inflammatory and anti-diabetic polyphenolic compounds like ellagitannins, anthocyanins, and punicalagins. Pomegranate extracts have been shown to inhibit adipocyte (fat cell) differentiation,[44] upregulate factors that discourage fat storage like peroxisome proliferator-activated receptors (PPARs),[45] and dramatically reduce the oxidative stress and inflammation associated with diabetes and obesity.[46]
A 2023 meta-analysis of 28 randomized controlled trials found that pomegranate consumption consistently reduces BMI, body weight, waist circumference, and fat mass. The meta-analysis calculated a weighted mean difference across all 28 studies, and found that on average, pomegranate supplementation produced a body weight reduction of 1.97 kg, corresponding to a 0.48 point drop in BMI.[47]
-
Green Tea Leaf Extract
Green tea extract is something of a metabolic silver bullet – its incredible range of antioxidant, anti-inflammatory, and antiglycemic effects is what earned it a spot in the (in)famous ECA stack for fat loss.
Research consistently indicates that green tea extract supplementation can improve:
- Fat oxidation rate and fat loss[48]
- Whole-body circulation[49]
- Insulin sensitivity and glycemic control[50,51]
- Blood pressure[52]
- Overall well-being[52]
Green tea extract is also great for defatting the liver, which, again, is absolutely crucial for maintaining metabolic health and limiting the damage from diabetes and obesity. One 2016 study found that people who supplemented with 500mg/day of green tea extract for 90 days had significantly lower liver enzymes.[53]
The catechin antioxidants in tea have been shown to significantly decrease both inflammation and fat accumulation in NAFLD patients.[54]
-
Grapefruit Fruit Extract
Grapefruit extract has powerful antidiabetic and anti-obesity effects thanks to its high content of citrus flavonoids like naringenin and hesperidin, which can help improve insulin sensitivity and decrease insulin resistance.[55]
A randomized, double-blind, placebo-controlled study from 2006 assigned participants to one of four groups for the 12 week study period – besides the placebo group, one got grapefruit juice, another fresh grapefruit, and finally, the fourth group took capsules continuing grapefruit extract.[56]
All the grapefruit groups lost significant amounts of weight. Although fresh grapefruit and grapefruit juice caused slightly more fat loss (1.6 kg and 1.5 kg on average, respectively), even the grapefruit extract group lost 1.1 kg in 12 weeks.[56]
The study also found that grapefruit consumption was linked to a notable reduction in postprandial glucose and insulin sensitivity.[56]
-
Bilberry Fruit Extract
Bilberry extract is a rich source of anthocyanins, a class of powerful antioxidants that have been shown to increase insulin sensitivity and improve glycemic control.[57] Research has shown that bilberry extract supplementation can increase glucose uptake by muscle cells, via GLUT4 upregulation,[58] and can also inhibit enzymes involved in carbohydrate digestion, which decreases the amplitude of postprandial blood glucose spikes.[59]
-
White & Red Grape Fruit Extracts
Note that red and white grape fruit extracts are not grapefruit – we are here talking about whole grapes of the genus Vitis.
Grapes are incredibly high in polyphenol antioxidants like resveratrol and flavonoids, which are known to increase insulin sensitivity and improve glycemic control.[60,61] Much like bilberry, grapes are also known to inhibit enzymes like glucosidase and amylase,[62] thus decreasing the absorption of dietary carbohydrate and reducing the size of postprandial blood glucose spikes.
-
Citrus Hesperidin
Hesperidin is a flavonoid antioxidant that's found in citrus fruits like oranges and lemons.[64]
Hesperidin upregulates cholecystokinin (CCK), one of the gastrointestinal enzymes related to GLP-1 activity (refer to the graph in our introduction to this article if you need a refresher).[65] Much like the flavonoids in Eriomin, it also upregulates PPARs, AMPK, and other insulin-related hormones like ACC.[66,67]
Hesperidin supplementation has been shown to improve serum cholesterol and fatty liver symptoms by down regulating cholesterol production.[68] It also seems to inhibit the creation of new fat cells.[69]
-
Chromium – 23 mcg (66% DV)
Chromium modulates the action of insulin, thereby improving insulin sensitivity and glycemic control.[70-72] The mechanism of action isn't completely understood, but seems to involve chromium's binding to a peptide complex called low molecular weight chromium binding substance (LMWCr). In turn, LMWCr can chelate chromium ions, creating a bioactive compound called chromodulin.[70,71]
One in vitro study demonstrated that chromodulin can increase insulin signaling by up to 800%.[73]
Wrapping up this ingredient profile, we find ourselves with an extraordinary profile of ingredients -- GlycoLemon, powered by Eriomin, is clearly no joke. Combined with the two fiber sources to support the satiety and appetite suppression many dieters want, and we have a powerhouse supplement.

Olive Leaf Extract brings a ton of benefits and is extremely popular for its weight loss and thyroid function benefits... but don't forget about immunity as well!
Dosage and Directions
Mix 1 packet with at least 8 fluid ounces of water, stir briskly, and drink immediately (the fibers will gel up if you wait too long)! Take once daily when you feel hungry between meals, or 30 minutes prior to a meal.
As always, maintain adequate hydration.
Incredible Beta Test Results: 28 People, 550 Pounds Lost!

Modere Curb's recent beta test saw 28 participants shed over 550 pounds in 90 days! Powered by GlycoLemon™ with Eriomin, Solnul® fiber, and glucomannan, Curb naturally supports appetite control and healthy weight management -- backed by real results and success stories!
Modere launched Curb on Saturday, September 21, 2024 with four simultaneous events. There, they explained their beta-testing, which included 28 participants (mostly women), who lost over 550 total pounds! They told their stories, which included life-changing journeys.
Modere also tracked data, including Akkermansia levels, which were elevated. You can read more in our detailed article on these results, "Modere Curb Reviews: 550 Pounds Lost by 28 People in 90 Days!".
We'll have more on this with our next podcast with Modere's CEO, Nate Frazier.
Part of the Modere Body Transformation Collection
Curb is part of a complete body transformation collection from Modere, which includes protein, CLA, a stimulant-based fat burner, collagen, and amino acids.
But in our opinions, the star of the show is Curb, thanks to the extraordinary efficacy of GlycoLemon, powered by Eriomin.
Conclusion: The Real Deal in GLP-1 Supplement Activity
Impressive. Beyond impressive.
With its powerful combination of Eriomin-powered GlycoLemon, and a unique blend of fibers and botanicals, Modere Curb sets a new standard for safe, stimulant-free weight management. By supporting GLP-1 activity, appetite suppression, and gut health, Curb stands out in a sea of weight loss supplements, offering a multifaceted approach to shedding pounds while enhancing overall well-being. As more data emerges, the possibilities for Curb's role in the weight loss industry will continue to unfold.
Stay tuned for further clinical and beta-testing data as Modere continues to innovate and refine its stack. For more insights, be sure to catch our next PricePlow Podcast with Nate Frazier, where we'll dive deeper into Modere's mission and future in the supplement world.
Nate knows what he's doing -- he actually launched Ghost at the 2017 GNC Franchise Show and locked down the Alani Nu exclusivity in 2019 when they only had Balance. He sees the future, and was on the front end of scaling many of the biggest launches in the past 10 years. This is big, and we can't wait to see what he has coming alongside it.
Don't miss out on our updates – check PricePlow's deals and sign up for our Modere news alerts today!
Modere Curb – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.


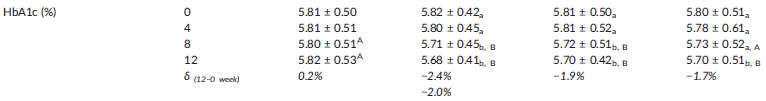












Comments and Discussion (Powered by the PricePlow Forum)