On April 25, 2023, Glaxon Founder and CEO Michael Bischoff was indicted on a misbranded drug charge stemming from activities of his company, G.B. Nutrition,[1] and on August 21, 2023, Bischoff pleaded guilty.[2]

On April 25, 2023, Glaxon Founder and CEO Michael Bischoff was indicted on a misbranded drug charge stemming from the 2016-2018 activities of his company, G.B. Nutrition. On August 21, 2023, Bischoff pleaded guilty. G.B. Nutirtion was later renamed to Zero Day Nutrition, and is the company that manufactures supplements for Glaxon (as well as many other companies). Glaxon is run by Day One Distribution at the same address as Zero Day, with G.B. Nutrition still listed as their registered agent in early March 2024.
This news has come as a surprise to us and, we expect, to nearly everyone in the dietary supplement space. Even though the case has already been prosecuted and a guilty plea and judgment has been entered as of August 21, 2023, we aren't aware of any public industry media coverage of this story.
Michael Bischoff Indicted on SARMs Charges in 2023
Below is the timeline of events, as found in publicly-available documents downloaded from PACER:
-
April 25, 2023: Indictment on One Count
The indictment was served with respect to G.B. Nutrition, Bischoff's company in Stafford, TX, covering activities between May 2016 and November 2018.[1]
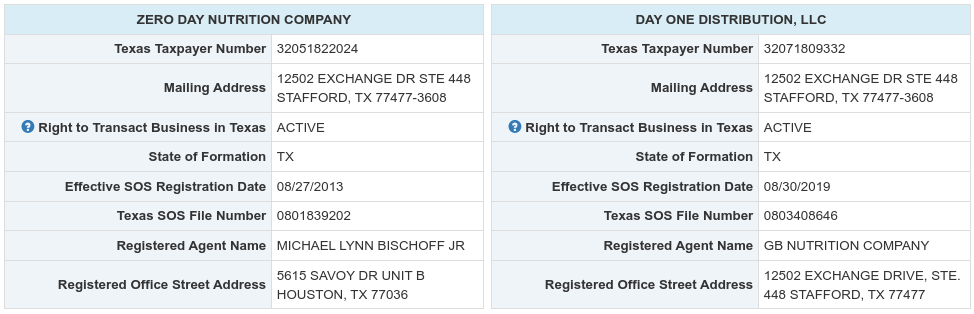
G.B. Nutrition is noteworthy because it was later renamed to "Zero Day Nutrition Company" on October 8, 2019,[3] an entity still used to manufacture dietary supplements for numerous brands. Among those brands is the popular supplement brand Glaxon, which is operated under Day One Distribution LLC, also owned by Bischoff. Day One Distribution lists GB Nutrition as its registered agent, and shares the same mailing address as Zero Day Nutrition in the Texas Comptroller's Public Records.[4]
Zero Day Nutrition, the company that was formerly known as G.D. Nutrition,[3] has the same address as Day One Distribution, the company that operates dietary supplement brand Glaxon. Day One Distribution's Registered Agent on file is even "GB Nutrition Company"! Data retrieved from the Texas Comptroller of Public Accounts on March 11, 2024.
After introducing the relevant laws and FDA regulations, the indictment states that:[1]
"Beginning on or about May 2016 and continuing to November 2018, Bischoff, regularly caused the introduction of misbranded drugs (SARMs) into interstate commerce, including, but not limited to, causing the introduction and delivery of introduction into interstate commerce the following misbranded drugs to Pennsylvania from the Southern District of Texas"[1]
It goes on to list two products made for Centurion Labz, labeled to contain SARMs (selective androgen receptor modulators) named Ostarine and Cardarine. These are notable because:
-
SARMs are not dietary supplements
SARMs do not fall under the definition of dietary supplements since they were not sold as dietary supplements before 1994, do not have an NDI (New Dietary Ingredient) acknowledgment from the FDA,[5-7] nor is there a known GRAS (Generally Recognized as Safe) affirmation to place them into foods.
-
Cardarine / GW501516
G.B. Nutrition was renamed to Zero Day Nutrition,[3] the company used to manufacture supplements for the popular brand Glaxon (which is operated under Day One Distribution).
Cardarine, also known as GW501516, was a drug collaboration between Ligand Pharmaceuticals and GlaxoSmithKline, but was abandoned because the drug was carcinogenic.[8]
-
Confiscations and Determinations
The products labeled as SARMs were confiscated, yet turned out not to contain the labeled ingredient. For instance, the cardarine product instead contained arimistane, according to the indictment.[1]
It's not entirely clear how the government considers this to be misbranded, given that there are different ways a supplement can be deemed as such. The indictment states that "They were misbranded in that the label was false and misleading."[1]
-
-
Forfeiture sought in other compounds
The government was seeking forfeiture for a very large amount of compounds, many of which are legal dietary supplement ingredients, but certainly not all. The forfeiture list in the indictment includes but is not limited to the following:
- Phenibut
- Noopept
- Adrafinil
- Boxes of bottles labeled "OSTA SARM", "Clomex", and others
- Two drums of DMHA
- One drum of DMAA
- One pallet of bags of white powder labeled "Malto dextrin made in China"
- Two bottles of Glaxon Pharm labeled products
-
May 11, 2023: Conditions of Release
A couple of weeks later, an order was posted setting the conditions of Bischoff's release on a Personal Recognizance bond.[10]
-
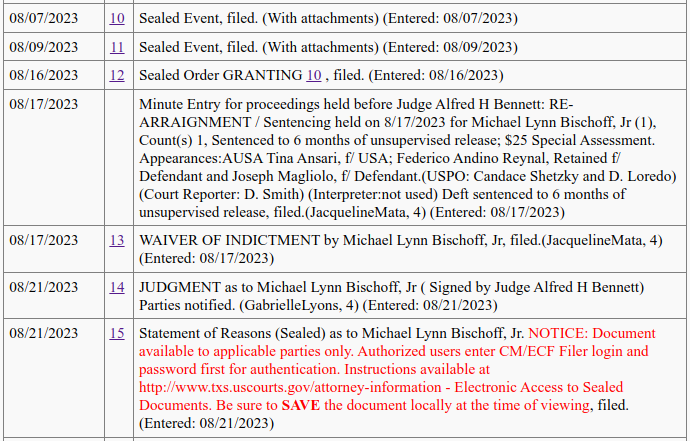
August, 2023: Sealed Events
On August 7, 9, 16, and 21, some events occurred that are sealed, and cannot be accessed:
-
August 17, 2023: Waiver of Indictment
On August 17th, 2023, Bischoff signed a Waiver of Indictment stating the following:[11]
I understand that I have been accused of one or more offenses punishable by imprisonment up to one year. I was advised in open court of my rights and the nature of the proposed charges against me.
After receiving this advice, I waive my right to prosecution by indictment and consent to prosecution by information.[11]
-
August 21, 2023: Judgment / Guilty Plea
On August 21, 2023, the courts filed a paper stating that the defendant pleaded guilty to 1 count on August 17, 2023 with the offense of Misbranded drugs into interstate commerce in violation of 21 U.S.C. §§ 331(a) and 352(a)(1). Bischoff was given 6 months unsupervised probation.[2]
There was also a sealed filing titled "Statement of Reasons" entered in the system on this date.
According to PACER, August 21, 2023 is the termination date, but one more action remained:
-
November 2, 2023: Destruction of Items Forfeited
On November 2, 2023, the judge signed an Order for Destruction, agreeing to the forfeiture and destruction of several misbranded drugs and other products listed on four pages.[12]
The final point above is of note, since Bischoff's brand Glaxon was not formally launched until 2019, but the indictment is for acts between 2016 and 2018. While there doesn't seem to be overlap from these two operations, they were both run out of the same corporate entity.
The criminal docket is for one count: introduction into Interstate Commerce of Any Drug that is Misbranded (21 U.S.C. §§ 331(a), 352(a)(1)).[1,9]
What will become of Glaxon?
It is unclear how this news will affect Glaxon and Zero Day Nutrition, since Glaxon was launched after the actions taken. Though the brand operates underneath a different company (Day One Distribution) than the one in the indictment (Zero Day Nutrition), they are clearly tied to each other in terms of manufacturing, their addresses, and even their registered agent on file as of early March, 2024.
Any news of Glaxon and its other employees will be updated through our Glaxon news page.
We'll keep this article up to date with any further developments on the Bischoff indictment, but aren't expecting many since the case is fully terminated.
The case ID is 4:23-cr-00181, served in the Southern District of Texas (Houston Division).
Article Updates: GNC's Statements after Removing (and Resuming Sales) of Glaxon Products
On March 12, 2024, GNC's Director of Public Relations reached out with the following statement:
"GNC is committed to the highest standards of product safety and customer trust. We are aware of the news concerning Michael Bischoff and the misbranding of products by his manufacturing company GB Nutrition between the years 2016 and 2018. We have no reason to believe that any products sold in GNC stores have been impacted as we brought the Glaxon brand into our locations in 2021. However, out of an abundance of caution, we will be removing all Glaxon products from our shelves effective immediately while we investigate and ensure that the product is safe and legal. While we have always trusted our partners to uphold the same standards of safety and integrity that we do, we are taking this immediate action to ensure the well-being of our customers. Our commitment to providing safe, quality products remains our top priority, and we will continue to take all necessary steps to uphold this promise."
-- Nick Sero, Director of Public Relations at GNC
Good news followed two weeks later, with his next statement:
After a comprehensive investigation, including independent product testing, a review of the FDA database, and confirmation of regular audits by the Texas Department of State Health Services, we have found no indication that Glaxon products were involved in any prior misbranding matters. Our findings have given us the confidence that all remaining Glaxon products meet label claims and contain no adulterants, reaffirming their safety and compliance with GNC standards.
Based on this thorough review, we have decided to resume selling Glaxon products in our stores starting Thursday, March 28. Thanks for your attention, and don't hesitate to reach out with any follow up.
-- Nick Sero, Director of Public Relations at GNC




Comments and Discussion (Powered by the PricePlow Forum)