So your YouTube fitness channel has swollen to 50,000 subscribers and it's time to monetize that supportive following. Perhaps you recently inherited some money, and you've always had an entrepreneurial itch that's as strong as your deadlift, or perhaps you just read the latest Gary Vaynerchuk book and think you see a new social media network ripe for a hostile takeover.
What's next?
Start a supplement company, of course!
Are you ready for what's next?
It doesn't take long before tragedy strikes. One day in the not-too-distant future, a fan of yours decides to up the ante by downing seven scoops of Pied Piper Pre Workout and finds himself in the emergency room.
This shouldn't surprise anyone, given that you were made 'InstaFamous' for ridiculous downing of multiple scoops of pre-workout in the first place. (Didn't exactly think that one through, did you?) Now the shoe's on the other foot and you're definitely NOT in a good position.
Here's what happens for YOUR new brand: An SAE (Serious Adverse Event) Report is filed with the FDA, and your former fan's parents are out for blood. They sue you for everything you're worth, get your YouTube channel shut down, and turn the media against you.
Here are the FACTS: This could have been prevented. If only you were more vigilant and got real serious about supplement safety.
Many startups in the dietary supplement industry-first believe that they can avoid the regulatory aspects of their business and do what they believe will help them steer clear of potential problems. Think about it. When you first launch a business, you're excited to get the word out - you want to spend money on promoting the business, not on adverse event surveillance.
So, you go at it and suddenly you're hit with an SAE (serious adverse event), which can realistically quickly shut that startup down. Of course, a savvy business person wants to spend money wisely and if you are wise, you'll instantly recognize how investing in the protection of your business and in the safety of your customers will be the best money you've ever spent.
It's time for some Nutravigilance Although extreme, stories like the one illustrated above can and certainly do happen. But more likely, there are a vast number of smaller situations (still referred to as Adverse Events) that require "adult attention" and can completely derail a growing supplement brand business.
People put this stuff in their bodies - it's time to start acting like it!
Many people think that the supplement industry is "unregulated", but the truth couldn't be any further from that. While it's true that the FDA does not require pre-market approval in order to launch a supplement — there are indeed a number of laws for supplement companies to follow. And at the end of the day, it's the brand owners' responsibility to follow them - not the manufacturer or the person slapping the label on.
After all, these are consumable products — as in, people put them into their bodies. So if you don't want to go the way of our Pied Piper example, it's time to take things seriously and get your regulatory & compliance affairs in order. That's where we come in...Supplement Safety Solutions to the Rescue!
Supplement Safety Solutions LLC is a physician-owned consulting company that helps its customers develop and refine standard operating procedures to ensure compliance with our complex Federal laws.[1] It's run by a pair of board-certified doctors who recognized the problems, having received the same countless Adverse Event calls from consumers - often after the damage was already done. The doctor team realized that it was high time for a standardized problem prevention service and monitoring solution for brands who were serious about their business.
The good doctors launched Supplement Safety Solutions, featuring Nutravigilance, a solutions-based program focused on supplement safety. This means consumer safety throughout the consumer consumption cycle – from that very first serving to the last-scoop, we assist by catching problems before they happen, and we assist with making sure that your brand's regulatory & compliance matters are fully buttoned up and prepared for a scaling business.
With the Nutravigilance program, Supplement Safety Solutions promises to help its clients exceed the current legal requirements with respect to safety and adverse event surveillance, monitoring, and reporting.
This is similar to the legally-mandated pharmacovigilance in the prescription drug world (collecting, detecting, assessing, monitoring, and preventing adverse events in pharmaceutical products),[2] but in the supplement industry instead!
But before we get into those unfortunate adverse events and the appropriate way to address them, we need to start from the top and discuss Supplement Safety Solutions and its two industry-renowned doctors.
The Doctors who Saved an industry & Paved the way Forward...
Bringing new supplements to the marketplace is your business.
Keeping them safe and protecting your brand is ours.
- Supplement Safety Solutions
Supplement Safety Solutions is run by Dr. Hector Lopez and Dr. Stephen Schmitz, both industry veterans. Dr. Lopez is a key opinion leader with a triad of companies that support human clinical research (CAHS-The Center for Applied Health Sciences, LLC) to substantiate the safety and efficacy of ingredients, finished dietary supplements, and functional foods. He also leads novel ingredient discovery, development, and licensing via NovaNutra and Ortho-Nutra.
Whichever supplement brand and products you consume, there's a great chance that Dr. Lopez had something to do with its success.
Dr. Schmitz has been a drug safety expert in senior pharmacovigilance positions within big pharma, but he has been a wellness medicine, dietary supplement research advocate, and Nutravigilance subject matter expert for almost a decade. We refer to Dr. Schmitz as Stephen "Adverse Event" Schmitz, considering he's reviewed more adverse events than anyone else on the planet.
Long story short. If you take a look at any of the dietary supplements in your cabinet, there is very likely at least one ingredient that one of these doctors has patented or helped bring to market. They are two of the "wizards behind the curtain", and that's where they typically like to remain -- making things better for those who are front & center, dealing directly with customers.
This is where Supplement Safety Solutions comes in:
The Services of Supplement Safety Solutions (SSS)
Supplement Safety Solutions provides a multitude of services aimed towards supplement and functional food companies and manufacturers; both big and small, to keep them legally prepared:
Pre-Market Supplement Services
- As doctors, the SSS team can help with human clinical research covering ingredients and/or finished product testing;
- IP and trademark protection - something we see companies repeatedly overlooks until it's far too late;
- Product claims substantiation and marketing content review. As you likely already know, label reviews are a critical element in your brand's business and must be properly dealt with.
In-Market Manufacturing Services
The Nutravigilance program and related services operate while your supplement is on the market:
- Helping define and refine SOPs (standard operating procedures) to ensure compliance with Federal Laws (especially the 2007 Non-prescription Drug and Dietary Supplement Consumer Protection Act[3,4]), the laws mandating an Adverse Event Reporting system -- ie. where Nutravigilance comes in;
- Providing additional compliance assistance with 21 CFR 111 (the current good manufacturing practices), and can audit and assist current QA teams, legal counsels, and existing GMP consultants;
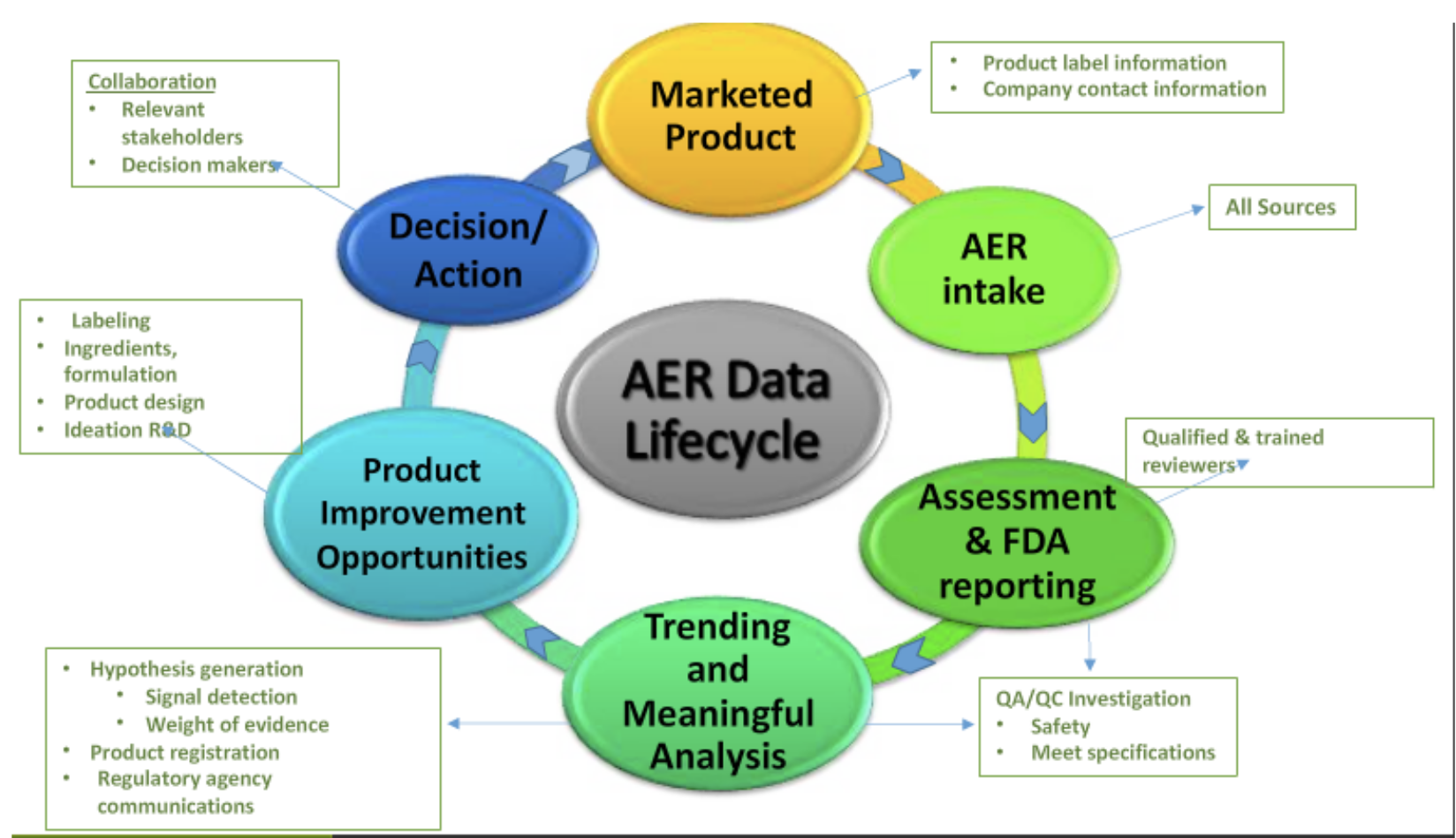
- Staying integrated and up-to-date with existing adverse event reporting data, spotting trends before supplement companies themselves (for instance, a trend of Adverse Event Reporting systems (AERs) with a certain ingredient that you may be already using or considering).
Post-Event Services:
Supplement Safety Solutions is also there for companies large and small in the unfortunate event that something does happen and it needs to be recorded and responded to legally:
- Adverse event assessment and follow-up;
- Customer follow-up and reporting of Serious Adverse Events to FDA through the Safety Reporting Portal (SRP);
- Complaint Log Review, Analysis, and bookkeeping;
- FDA inspection preparation and conduct assistance;
- FDA Warning Letter response (to FDA Form 483).
As you can see, the doctors at SSS are extraordinarily well-prepared and well-connected with the right researchers and attorneys to cover all facets of a truly legal supplement brand launch and run, from start to finish.
But the area where too many brands get caught with their pants down is in the case of adverse events, and that's where Nutravigilance comes in:
The five key concepts of adverse event reporting
Nutravigilance was based upon a study published in 2014 that outlines the five following principles:[5]
- Key concept 1: The Company is responsible for demonstrating oversight on product safety.
- Key concept 2: The Company has written procedures that cover adverse event reporting to meet the requirements of the AER law.
- Key concept 3: The responsible person is the key regulatory contact.
- Key Concept 4: The company ensures safety data is regularly evaluated to detect safety signals.
- Key concept 5: The company develops and updates a quality management system for cGMP and adverse event reporting.
Dead Brand Tell No Tale! If you don't know what the hell we're talking about, yet, and you run a supplement company, then you are not ready.
Nutravigilance: exceed current regulatory requirements
Supplement Safety Solutions was fielding too many calls in the aforementioned "after the fact" situations and realized that too few companies were prepared for adverse events. Many companies aren't even aware of the actual laws regarding event reporting (ie the 2007 Non-prescription Drug and Dietary Supplement Consumer Protection Act[3,4] as mentioned above).
So they came up with a standardized program dedicated to continuously monitoring and protecting their consumers, making sure to cover the key concepts identified above — and that program is called Nutravigilance.
With Nutravigilance, brands fill a major regulatory compliance gap that often goes unmonitored, protecting them in several "worst-case scenarios". It is a full-service AER-SOP program and provides coverage where the government requires it.
The program also gives brands access to the right consultants and the right legal teams in the case of any other situation.
Do you see the Nutravigilance seal?
The Nutravigilance Verified seal[6] on product labels and marketing materials shows customers that a quality third-party is actively monitoring any consumer complaints or reports of potential health problems related to (or even associated with) — but not necessarily caused by — the use of a supplement.
This is a "good steward assurance" seal that shows a brand is not only meeting the minimum mandatory standards of federal and state consumer protection laws but exceeding them for both product complaints and adverse events reporting.
In an industry with many "bad actors," this seal is one more way to prove a brand is doing it right and is connected with the "good guys."
So if you're serious about your supplement brand business, and want to stick around for the long haul, it's time to get a bit more vigilant about the laws and let the team at Supplement Safety Solutions/Nutravigilance smoothly pave your way forward.









Comments and Discussion (Powered by the PricePlow Forum)