1-Andro (1-DHEA) is one of the popular legal prohormones of 2016 and has a legit human study on it with serious serious steroid-like gains... but you must do your homework here before you get involved!
Back in my day, prior to the ban in 2005, we had a variety of great prohormones and designer steroids on the market that were available for sale legally. They were either found in nature and thus legal under DSHEA, or there were loopholes in the law allowing for their legal sale.
But times have changed, and in response, the industry has changed as well...
TL;DR
-
1-Andro is a legal prohormone (or more specifically, a preprohormone) that gets converted to the active steroid 1-Testosterone
-
Human research has shown true steroid-like gains: researchers noted that it's as effective as a weekly cycle of 300mg injectable testosterone enanthate!
-
Dosage and the recommended 1-Andro product depends on binding and delivery systems, as well as user experience:
-
The steroid-like gains were found with daily doses of 330mg 1-Andro combined with 150mg 6,7-dihydroxybergamottin (grapefruit extract) -- exactly what's provided in a day's dose of Hi-Tech Pharma's "1-Testosterone" product.
-
First-time 1-Andro users are wise to use lower doses. Hi-Tech Pharmaceuticals "1-AD" contains 75mg 1-Andro Decanoate (ester-bound), yielding 47mg 1-Andro, which beginners should take twice per day.
Yet more is not always better. The body can only convert so much 1-DHEA into the parent hormone. Much higher doses could mean you're just throwing money away and increasing the risks of negative side effects.
-
-
A prescription-grade post cycle therapy (PCT) is required. Estrogen can potentially be elevated, and natural testosterone production will decline.
Because of this, you must see your endocrinologist before starting any cycle, and do not use any prohormone without a doctor's consent!
-
Our minimum recommended age is 23 years old, although the "official" recommended age is 21+.
-
Side effects are consistent with steroid use: increased liver, kidney, and cardiovascular stress, although they are temporary and returned to baseline after cessation. Users are still highly encouraged to take a cycle support supplement to combat these sides.
-
Due to its aggressive nature, we do not recommend 1-Andro as your first ever prohormone - more experience is required.
Easier alternatives are 1,4DHEA provided by Hi-Tech's Equibolin. Future articles will detail this prohormone.
1-Andro is also known as 1-DHEA and 1-Androsterone, terms we use interchangeably in this document. Don't confuse them with the compound 1-Testosterone, which is what this converts to.
Continuing with our history lesson, since we need to understand what we're trying to replace and re-create:
When 1-Testosterone was king
One of the best compounds in this now-banned class of prohormones is named dihydroboldenone. Since that's challenging to say, it was given the marketing-friendly term of 1-testosterone, which is the name that stuck for the steroid. It was renown for one of the most potent anabolic steroids sold over the counter, and was used by countless bodybuilders and enthusiasts to build significant amounts of lean muscle mass without having to end up giving Rick Collins a call for getting busted with the illegal stuff.
2005: The FDA and DEA take away 1-testosterone
Unfortunately, the killjoys at the FDA and DEA decided that we can't be trusted with our own bodies, and ended up banning the stuff, along with all the other legal prohormones and designer steroids under the Anabolic Steroid Control Act of 2004, which went into place in 2005.[1] With that law in place, 1-testosterone either vanished entirely or was relegated to the black market.
Prior to the ban, there were several ways to use 1-testosterone. Transdermals (topical skin application) were very popular, except for the fact that 1-testosterone was known to be an irritant and could cause a rash. Some manufacturers also bound 1-testosterone to an ester and sold it as a liquid gel cap, with mixed results.
1AD: Formerly the way to go...
But one of the most popular formulas was actually a precursor to 1-testosterone, known as 1-Androstenediol, or 1AD. 1AD was a one step conversion through an enzyme pathway to convert to 1-testosterone in the liver. This formula was highly bioavailable orally and worked very well for most that tried it.
That, too, was unfortunately banned in 2005.[1] So now what?
Bringing back 1-Testosterone: Build your own! (after taking 1-Andro)
What seemed to be a nostalgic memory has been brought back from the dead with the development and sale of a preprohormone of 1-testosterone.
1-testosterone has been "reintroduced" as a precursor to a precursor, if you will, in the form of a compound most commonly known as 1-Andro (yet with several other chemical names listed below). This compound is a modified form of the parent steroid hormone DHEA that takes a two-step conversion to 1-testosterone via two enzyme pathways,[2] discussed in detail below. It looks promising both on paper and in the study that utilized it in trained males.
First, however, let's discuss 1-testosterone in more detail, since this is what we're targeting with 1-Andro anyway.
How 1-Testosterone Works
This section takes a step backward, before we get into 1-Andro:
1-testosterone should actually be known as dihydroboldenone, which is what the steroid boldenone converts to when it interacts with the 5 alpha reductase (5AR) enzyme. Similar in the way that testosterone can convert to DHT (dihydrotestosterone), when 1-testosterone interacts with 5AR, the 1-testosterone is then modified in such a way that it can't convert to estrogen.[3]
Because 1-testosterone is 5AR reduced, unlike regular testosterone, it doesn't make conversion to DHT via 5AR in the scalp and the skin. Although there are still a significant amount of androgen receptors in both of these locations, 1-testosterone should be theoretically less apt to have an impact on those sites. Nonetheless, 1-testosterone is a highly androgenic (and anabolic) compound and the usual side effects such as acne and androgenic alopecia can still be a concern for those susceptible.
![1-Testosterone Synthesis: So our goal is to get a boost of 1-Testosterone in our systems. This pathway using 1-Andro is one such way to get there!<sup>[4]</sup>](https://blog.priceplow.com/wp-content/uploads/1-testosterone-synthesis-300x447.png)
1-Testosterone Synthesis: So our goal is to get a boost of 1-Testosterone in our systems. This pathway using 1-Andro is one such way to get there![4]
The fact that 1-testosterone can't convert to estrogen is a plus for those looking for more lean or "dry" gains or people concerned with common estrogenic side effects such as gynecomastia (gyno) or bloating.
The downsides of 1-testosterone
On the negative side of things, 1-testosterone had the reputation for causing lethargy and tiredness in its users, the cause of which has never really been determined, although there's been much speculation as to why. There was also anecdotal reports that some users reported of loss of libido on 1-testosterone, but again this wasn't reported in every user and was never confirmed in any study.
Some steroids just appear to impart effects on the body beyond the androgen receptor, and due to a lack of research studies on AAS, all we have is "broscience" to attribute these to -- whether they even exist or are purely placebo effect is still unknown.
The past fixes won't work today
In order to combat those issues in the past, 1-testosterone and 1-androstenediol (1AD) prohormone users would add 1AD's chemical cousin, 4-androstenediol (4AD) to the stack. This would mimic what most AAS users do, by creating a cycle around a base of testosterone and then adding compounds to that depending on the results they were looking for.
330mg daily of 1-DHEA was comparable to a cycle of 300mg a week of injectable testosterone enanthate!
Unfortunately for us today, we don't have legal access to 4AD, but only its preprohormone of 4-DHEA. The problem is that since 1-DHEA (the eventual subject of this article) and 4-DHEA convert to their parent hormones via the same enzyme, it's highly likely that taking both in combination would cause those enzymes to become oversaturated. This would then leave the user with not only subpar results, but much less conversion to the hormones they're wanting to elevate.
The new lessons to be learned
Enzyme conversions of prohormones to their parent hormones only have a finite ability. For example, at high enough doses of 1-DHEA, there's a point where your body will be unable to convert any more 1-DHEA into 1-testosterone. And unlike anabolic steroids, you simply can't fix this by consuming more of the compound.

The Anabolic Steroid Control Act of 2004 forced the industry to react, and the latest technology puts us in a place where we can get what is needed to product 1-testosterone, but there are caveats...
So what we're trying to say is that if the 1-DHEA discussed later on in this document imparts tiredness and/or loss of libido, there is no current legal hormonal equivalent to do what worked in the past. The good news is that these sides are not at all guaranteed, as we'll see in the research, where mood was actually elevated by 1-DHEA.
We're also saying that you're not going to want to stack full doses of 4-DHEA with full doses of 1-DHEA since they compete for the same enzyme.
All in all, back in its heyday, the pros of 1-testosterone outweighed the cons for large numbers of hardcore users. After the ban, this sent the sports nutrition world on a hunt to find something that is legal and can yield some of the same properties and effects by converting to it.
Therein lies 1-Androsterone:
The 1-Andro Research: How it works
As mentioned before, 1-Andro takes a two-step conversion (via two enzyme pathways) to get to 1-testosterone.
There has only been one formal study on 1-DHEA, titled "Prohormone supplement 3-hydroxy-5-androst-1-en-17-one enhances resistance training gains but impairs user health" from 2013.[2] Although the sample size in the study was relatively small, utilizing only 17 men (aged 18 to 35), the results were quite significant. The dosage used in the study was 330mg of 1DHEA per day - spread across three doses each day with a meal per day for 4 weeks total.
The study went into detail regarding how 1-DHEA is metabolized into its parent hormone:
Upon ingestion, 3-hydroxy-5-androstan-17-one is sequentially converted by 3-hydroxysteroid dehydrogenase and 17-hydroxysteroid dehydrogenase enzymes to yield 17-hydroxy-5-androst-1-en-3-one (1-testosterone).[2]
This indicates that 1-Andro is indeed a preprohormone converting via two steps into it's parent hormone, 1-testosterone.
The study indicated that the group receiving the dosage of 1-Andro increased their lean muscle mass, lost fat mass, as well as increased strength in their back squat, bench press and deadlift.
Steroid-like gains - Seriously, this time!
One of the most remarkable points brought up in the study was how the authors compared the usage of 1DHEA to actual steroids -- and the muscle growth, strength increases, and fat loss found when using those compounds.
[W]e feel the reader would benefit from a comparison of the effects we reported with po (oral) PS (prohormone supplements) administration to prior work that examined the same effects in participants who received intramuscular (im) testosterone enanthate administration.
In one such study, subjects received a long-acting gonadotrophin releasing hormone agonist to block endogenous testosterone administration and weekly im testosterone enanthate treatments over a 20-wk period. Testosterone enanthate at a dose of 300 mg/wk contributed to a doubling of subject's baseline circulating testosterone concentrations and increased their fat-free mass and leg press strength by 8.2 and 19.5%, respectively. These adaptations were noteworthy, as they came in the absence of resistance training.
More recent work by Rogerson et al. examined the effect of combining 3.5mg/kg weekly doses of testosterone enanthate with a 6-wk resistance-training intervention. The body weight of subjects in that study averaged 79.2kg, making the average testosterone enanthate dose used (277 mg/wk) very similar to the 300mg dose that we reported on in the prior reference. In the Rogerson et al. study, the combination of testosterone enanthate and resistance training contributed to a 6.4% increase in body mass and a 15% increase on both bench press and leg press exercise.
We find it interesting that the improvements reported in both of these studies are remarkably similar to the improvements we report here, where 4 wk of daily po administration of 330 mg 3-hydroxy-5-androst-1-en-17-one [1-DHEA] and 150 mg of 6,7-dihydroxybergamottin contributed to a 6.4% increase in fat free mass and 9.2, 14.2, and 14.6% increases on the bench press, back squat, and deadlift, respectively. Collectively, these studies suggest that [oral] administration of 330 mg 3-hydroxy-5-androst-1-en-17-one/day provides improvements in body composition and muscular strength that are similar to those shown when 300 mg/wk testosterone enanthate is administered IM.[2]
To summarize, the authors found that the administration of 330mg daily of 1-DHEA was comparable to a cycle of 300mg a week of injectable testosterone enanthate! That is an incredible statement to make, and if correct, would put 1-DHEA on the same level as anabolic steroids available on the black market!
Mood improvements, not "roid rage"
The authors also interestingly noted that they observed users of 1-DHEA had positive changes in their psychological output with an increased sense of wellbeing and improved productivity.[2] A very novel finding, to say the least, with the demonization of AAS and their notorious reputation of large angry men with "roid rage".
1-Andro Side Effects
So far everything's been great, but what about the downsides?
As with all anabolic hormones, there will be the typical side effects potential of acne, hair loss, prostate enlargement, cardiovascular problems (such as high blood pressure), increased liver and kidney values, and testicular atrophy, amongst other things.
With pure 1-Andro, we have direct evidence of this, as established by the 330mg study. In fact, it as so much to the point that they titled the study "Prohormone supplement 3-hydroxy-5-androst-1-en-17-one enhances resistance training gains but impairs user health".[2]
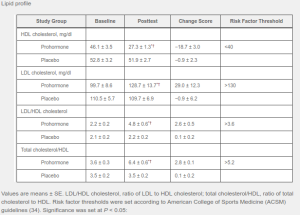
1-Andro Side Effects: Beyond liver and kidney issues, 330mg 1-Andro (1-DHEA) caused healthy lipid levels to deteriorate (HDL is the good cholesterol). The good news is that levels returned after prohormone cessation, but we still strongly suggest an on-cycle support supplement as well as a prescription-grade PCT. Whether it's possible that a better delivery system that uses less active ingredient is any better is to be determined...
For instance, various negative side effects were noted including significant elevations of LDL (bad) cholesterol, a reduction in HDL (good) cholesterol. There were also increased levels of aspartate transaminase, indicating 1-DHEA is taxing on the liver by some amount.
Kidney strain was also noted with noted markers being demonstrated of the kidneys working in overtime to process and filter the substance out of the body.
Dealing with some of these effects
To some extent, these problems can be reduced by using a smaller dose, using a short cycle timeframe, and including extra supplements such as "On Cycle Therapy" that contain compounds like NAC to help detoxify the liver, trans-resveratrol for the heart, and others.
However, the study authors themselves noted in regards to the negative cardiovascular effects:
However, we must also acknowledge that lipid profile changes induced by short term anabolic usage are known to self-resolve when anabolic usage is discontinued.[2]
They go on to say about the kidney health concerns:
Thus the likelihood that PS (prohormone supplement) subjects experienced kidney damage is minimal.[2]
That isn't to say that there's nothing to be concerned about, because the authors mentioned that the observations they made were in line with changes that were to be expected during AAS usage and should most likely return to baseline after the cycle.
It goes to illustrate the very important need to have blood work done BEFORE and AFTER a cycle! If you already have undiagnosed health issues, such as liver and kidney problems, and stress them out further by using 1-DHEA, it could be a recipe for disaster.
Not a methylated steroid, but behaves like one?
What's interesting about the negative health results with this compound is that it is not a methylated steroid, yet is exhibiting some of the effects on the liver and kidneys you would see in these types of steroids. It's worth asking whether or not 1-DHEA is 'safer' than it's predecessor or even 1-testosterone used alone.
In the end, these are hypothetical questions that a user will just have to take into account when deciding to use this compound. At the end of the day, this is currently what's here and what's legal right now (March 2016).
Understanding and learning from the numerous byproducts
The fact that there are so many byproducts reinforces our previous urging to get blood work done before and after the cycle
Another interesting point of interest is the list of multiple byproducts and related androgens that 1-DHEA breaks down to while it's going through its transition into 1-testosterone via the two enzymes, 3-hydroxysteroid dehydrogenase and 17-hydroxysteroid dehydrogenase.
When looking at a study performed by The Center for Doping Research in Germany, "Seized Designer Supplement Named '1-Androsterone': Identification as 3-hydroxy-5-androst-1-en-17-one and its Urinary Elimination",[4] we are able to see the entire pathway that 1-DHEA takes when it's converting to 1-testosterone, and it's quite a remarkable chart (shown below).
According to the study, 1-DHEA converts to an astounding twelve different 4-carbon steroid compounds, one or more of which may convert to estrogen and can each have different routes of conversion to other compounds on their own. To cover each and every byproduct that 1-DHEA converts to would be far beyond the scope of this article, a few notable byproducts include 3α-Androstanediol, a metabolite of DHT that appears to benefit the mood. In addition, there's epiandrosterone, another product sold as a prohormone that converts to DHT. DHT is yet another metabolite in the conversion product of 1-DHEA.
![1-Andro Metabolism: A study has shown that there are twelve byproducts of 1-Andro's metabolism.<sup>[4]</sup> Many of them anabolic, but some may be estrogenic. Blood tests are highly encouraged. Credit to shortythree on the bodybuilding.com forums for helping decipher some of these molecules! *We believe compound (6) is related to 1AD but isn't exactly the same thing.](https://blog.priceplow.com/wp-content/uploads/1-andro-metabolism-625x398.png)
1-Andro Metabolism: A study has shown that there are twelve byproducts of 1-Andro's metabolism.[4] Many of them anabolic, but some may be estrogenic. Blood tests are highly encouraged. Credit to shortythree on the bodybuilding.com forums for helping decipher some of these molecules! *We believe compound (6) is related to 1AD but isn't exactly the same thing.
But one of the most notable byproducts is that 1-DHEA converts to 1-androstenedione. The dione versions of androgen precursors can convert to estrone via the 19-Hydroxy-androstenedione pathway and then on to estradiol, as well as converting to estriol through another set of enzyme pathways.
All of this begs the question as to whether or not these metabolites are interacting in a major way to either impart gains, negate gains, increase side effects, reduce recovery times by further suppressing the hypothalamic pituitary gonadal axis (HPTA axis) or any number of other things. On the other hand of the argument, are they converting to an appreciable degree to make a difference in the slightest, or is 1-testosterone the principal conversion hormone here and all these other byproducts are broken down and completely ineffective?
Without in depth studies on 1-DHEA we will probably never know the answer to these questions, and sadly, this is one of the few prohormones that actually has any research.
In the end, it doesn't matter all that much to the average bodybuilder who is using 1-DHEA to impart muscle gains or fat loss, since the gains ultimately came in a major way.
But the fact that there are so many byproducts reinforces our previous urging to get blood work done before and after the cycle, keep an eye on your blood pressure, and pay close attention to any estrogenic or androgenic side effects that may pop up during the cycle to ensure your health remains good throughout the course of the cycle.
Who is it for
1-Andro/1-DHEA is for experienced individuals looking to add more lean muscle mass or to preserve muscle mass during a fat loss or cutting cycle. As with all anabolic hormone products, it's only for advanced users who known proper methods of weight training and diet to get the most from this product and know how to cycle off.
Come with prohormone experience

Never used a prohormone before? 1-Andro is a bit aggressive for beginners... you might be better off with 1,4DHEA in Equibolin instead.
Throughout our research on 1-Andro, we've come to the conclusion that this should not be your first prohormone, meaning that you should have experience with others -- such as 1,4DHEA (from Hi-Tech's Equibolin) before stepping up to this plate.
This leads us to our next section:
Who is it not for?
Again, this is not a beginner's compound. 1-Andro not a standard "food supplement" in the sense that it's boosting something you already eat (like protein or creatine). It's a hardcore tool for guys who are at the advanced level. Not to mention that it would be a waste of money for rookies, since beginner gains can often exceed those of anabolic steroids and that is no exaggeration!
Men with any type of cardiovascular health problems, prostate issues, high blood pressure, liver or kidney problems would also want to avoid this. Men who are planning on having a family within 6 months to a year should avoid this compound during this time frame, as anabolic hormones are known to suppress sperm production.[5]
What age?
The "official" stance is that anyone under the age of 21 should avoid this and focus on learning the basics of nutrition and weight lifting before embarking on cycles of anabolic hormones.
However, our stance here is that anyone under the age of 23 shouldn't take this.
Why? There are three answers, the first of which is most important:
-
Your growth plates are not yet done fusing.
Growth plates fuse via estrogen.[6,7,8] Typically our growth plates (in the spine and hips) are done fusing and finally close at around 22-23 years old[9] (editor's note: Mike, the founder of PricePlow, sprung an extra inch between ages 21-22).
So, when anyone younger than that starts using hormonal compounds, there is a chance that estrogenic compounds are going to cause them to potentially stop growing any taller than they naturally would. If there is even a remote chance of this, trust us, it's not worth taking. Seriously.
But do these new ingredients aromatize?
This part can be debated, but it appears that these ingredients do aromatize. In the study discussed above,[4] we see that it converts to 1-androstenedione as well as the 1-androstenediol version.
Back in the day when it was legal, it was preferred to use the DIOL versions of prohormones because they would only interact with the enzyme to convert to their parent hormone, and not back to estrogen. However, the DIONE versions would convert back to estrogen. See the sidebar, which discusses this in greater detail.
For this reason, the unfused growth plates are at risk. If someone is in that 21 year age range and thinks they might be able to eek out any more growth, they can run a prescription-strength aromatase inhibitor alongside the 1-Andro to make sure estrogen stays low throughout the entirety of the cycle. This will require a prescription from an endocrinologist.
The caveat side: some research claiming BS
Then again, to be fair, another popular study questions the entire above argument, stating that it might be BS.[12]
We'll always try to give you two sides of the story on things like this, but ultimately, is this worth the risk to young guys? We say no.
-
Younger guys already have testosterone levels and GH levels that are sky high
...and don't have enough knowledge with diet and training that they would benefit from using AAS at 18. Don't be overconfident here, there is still a ton to learn and gains take time. You can't have it all yesterday.
This life lesson is free: stay disciplined -- not just "motivated". If you're a lifter, you're in for the long haul anyway.
-
The mental side
It is easily argued that younger men cannot (and do not) handle any mental/anger effects from hormones. There's no science to back this up, but there are too many ugly stories to ignore. The caveat on this one being that 1-DHEA seems to improve mood for adult users, with the study showing only a very small increase in anger (0.1) in their mood profiling.[2]
While we're giving both sides of the argument, it's worth stating that there's very little difference in the endocrine system of an 18 year old in comparison to a 21 year old. But that's not the concern here - the concern is with your height and training abilities.
So at the end of the day, it's all about those growth plates. Like it or not, the world is what it is, and every inch of height counts - nearly to an exponential degree (ie it's ten times better to be 5'11 than 5'10, and it's ten times better to be 6'0 than 5'11). Taking a PH at age 18 simply isn't worth it from the long-game perspective. Pay your dues and wait your time.
At PricePlow, we're still conservative about actually using them. 23 and over only please.
Drug tested? Not a good idea...

Drug-tested athletes: Don't even think about it!!! (As far as employers go, check with your management. This is legal in 2016 so we hope it doesn't have bearing on employment).
For those concerned with, *ahem*, how long the substance lasts in the body before it will bypass a drug test result, our one study showed a single 115mg dosage was still able to be detectable in the body for a full 7 days. So this is likely not one to gamble with, since it may continue to last well beyond that. Not to mention, the German study included a list of metabolites that can be found when consuming 1-DHEA, so it's likely anti-doping organizations have already caught on to this compound and can detect it easily.
Trying prohormones for the first time? 1-Andro might not be your best option
In addition to the above statements, we're not going to recommend this to anyone who's never run a prohormone before. Getting more 1-Testosterone into your system is the real deal, and it can bring on some side effects, as discussed above and below.
Instead, we think that 1,4DHEA would make for better starter prohormones. So if this is your first run, we'd rather you consider Hi-Tech's Equibolin (1,4DHEA).
These ingredients and products will be discussed in greater detail in the future, and we'll link to them from here when ready.
1-Andro Dosage Considerations
The only study that looked at 1-DHEA used a dosage of 330mg per day to achieve results of around 4kg (8.8lbs!) of lean muscle over 4 weeks. Yet it's not always so simple.
...but it depends on the binding and delivery system

Cyclosome is a new supplement ingredient delivery technology made by Hi-Tech Pharmaceuticals that combines the forces of two other delivery systems. This should make for prohormones that require smaller doses
The version used in the study was plain 1-DHEA taken orally and did not use any kind of advanced delivery system, which could make a difference as to whether less could be used.
That study did however include 6',7'-dihydroxybergamottin (DHB) in the 1-DHEA formula. This compound is an extract of grapefruit that is meant to inhibit liver enzymes that would typically breakdown hormones.[13] With this inclusion, it likely allowed more 1-DHEA to circulate in the body, but then also led to increased stress on the liver. The Hi-Tech 1-Testosterone prohormone contains the same amount of 1-Andro and 6',7'-dihydroxybergamottin found in the study.[2]
This takes us to the next section -- which products have 1-Andro, and what we recommend:
The best 1-Andro product
So what's the best 1-DHEA product to use? There are two ways to go here, and it will depend on your experience level.
First time? Go with 1-AD by Hi-Tech Pharma

First time 1-Andro user? Smart to start with Hi-Tech Pharma's 1-AD (which does not contain 1-Ad -- it contains 1-Andro!)
With new age delivery systems such as Hi-Tech Pharmaceutical's Cyclosome, the "beginner" dosing of their product "1-AD" is 75mg twice per day - with a max dose of four tablets per day. Realize that they are using an ester-bound version of 1-Andro (which yields better pharma-kinetics), so per our calculations, you're ultimately getting about 47mg actual 1-Andro per tablet.
This means that a max recommended dose of Hi-Tech's 1-AD will be roughly 188mg, but given that straight 1-DHEA used in the study probably has extremely low absorption (3-6% bioavailability in standard DHEA),[14] and the two technologies inside Hi-Tech's Cyclosome process may yield anywhere from 9% to 90% bioavailability depending on the compound, we're extremely confident that everyone should start at two tablets per day, yielding 150mg active ingredient or 94mg 1-Andro - far more should get absorbed.
For a first-time 1-DHEA user, this would be a great place to start.
Take 1-AD (or any esterified prohormone) with Food
As a note, a study on oral testosterone undecanoate showed that all results were far better in subjects who took their dose with food, as opposed to fasted.[26]
This leads us to believe than any ester-based steroid or prohormone, Hi-Tech's 1-AD included, should be taken with food.
Hi-Tech's "1-Testosterone" Product: More advanced, more study-replicated
1-Testosterone has the 330mg/day dosage in the study, plus even the grapefruit extract. Once again, don't be fooled by the name - it's a 1-DHEA product!
A higher dose for more advanced users is the 1-Testosterone prohormone by Hi-Tech Pharma, which also includes the Cyclosome delivery system, yet has 110mg 1-Andro and 6,7-dihydrobergamottin, as mentioned above. So you'll definitely get more bang out of this one.
Better absorption: grapefruit vs. ester?
In terms of absorption when comparing Hi-Tech's 1-Testosterone vs. their 1-AD, it will depend on what works better - the ester (which steals some space in 1-AD) vs. the grapefruit extract.
Note: Don't be confused by Hi-Tech's product names. 1-Testosterone is a prohormone with 1-Andro inside. Same goes for their 1-AD. They of course convert to 1-Testosterone in your body, but you are buying *1-Andro* with these! They're just clever marketing names!
Other products containing 1-DHEA
Note: This area has been updated to reflect what's in stock. The Hi-Tech Pharma brands and products above are the ones that are typically in stock.
- Blackstone Labs Chosen 1 - Uses 65mg of 1-DHEA, 1-DHEA Undecanoate, and 1-DHEA Caprylate
All brands/products that have carried these recently (APS, Blackstone Labs, LG Sciences, and Innovative Labs) are in the Hi-Tech Pharma family. All of their new products will have the high-absorption Cyclosome delivery system.
These are literally the only two companies we trust producing these prohormones at this time.
Cycling Questions
Immediately after publishing this article, we were asked, "Will I have to cycle on and off whole life?"
Our answer is below:
Not necessarily. For example, you could do a 12 week cycle of AAS and gain maybe 15lbs of muscle and expect to keep 10 of those PCT as long as you keep training, intaking protein, etc.
What a lot of bodybuilders find though, is that they have (ab)used steroids for so long that their HTPA axis finally just shuts down permanently and they have to go on TRT therapy the rest of their lives just to maintain their test levels.
It comes down to a question of the person's preference and lifestyle goals. If they want to keep increasing the size of their muscles and get up to a 300lb competitive bodybuilder, then yeah, they're going to have to cycle on and off forever essentially. But, if you're just looking to add maybe 30lbs in a year you could do 2-4 cycles a year and still maintain the majority of that muscle mass.
Beware high doses of the standard 1-DHEA
Going back to straight 1-DHEA, an advanced steroid user could possibly get away with going a bit over 330mg amount (never more than double) and have serious results, but likely have quite serious negative side effects in terms of liver, kidney, and lipid levels.
We hope to avoid that situation - we firmly believe a far smaller dose of a liposome-encapsulated version is magnitudes safer -- especially for first-time users who should use the ester-bound preparation in HTP's 1-AD product.
Even besides the issues of side effects at higher doses, more still doesn't always mean better:
High-Dose Issue: Two pathways that can get saturated
There is also a question of enzyme saturation when using steroid precursors. Because of the fact that this compound requires two enzyme pathways to make the full conversion to 1-testosterone, either of those enzymes can become saturated and unable to convert anymore into it's parent compound - regardless of how much is taken.
Since 1-DHEA is relatively new and the fact that there is only one clinical study on it, it would be wise to browse popular forums around and read feedback from other users and make up your own mind on the dosage that might be right for you, ask to see blood work from regular users (and not supplement reps), and get a general grasp of how is affecting some people.
Since hormones are very slow acting in the body and can take weeks to build up and start to have effects, it may be hard to judge how they are working by feel alone. This is why feedback from the community might be of benefit to you, nonetheless, if you're not sure, the 300mg per day dose appears to be mostly safe for healthy users who use proper ancillary compounds and monitor their health in a 4 to 6 week time frame.
Long story short: Take two tablets per day of the good stuff (Hi-Tech's)
So the question comes down to the superior delivery systems for 1-DHEA that allow it to get past the stomach acids and not be broken down and destroyed by the liver. As mentioned above, we're sticking to the recommendation of Hi-Tech's products: starters should start with their 1-AD, and more experienced users can go right to their 1-Testosterone.
Follow the label's instructions closely. For 1-AD, that means two tablets per day for your first run, but max out at four tablets per day after you've run a cycle.
Transdermal products: not enough information
As an FYI / addendum, a topical or transdermal system could also be worth investigating in the future, depending on whether the 1-DHEA molecule is small enough to pass through the membrane of the dermis and if the transdermal formula includes well formulated ingredients that can help it pass through the layers of the skin.
We don't have enough info on this but may update this post if more comes to light.
In order for a molecule to pass through the skin via a transdermal formula, the molecular weight of the compound must be less than 300 so it can make it through the skin unabated. The formulator of the transdermal product must also know what they're doing and use proper compounds that allow it to permeate the skin, as well as bypass first-pass metabolism of the drug, allowing more to get into the system rather than an oral delivery system.
This gets into a completely different topic of discussion, however. If one were so inclined and had access to the bulk powder hormone, it would be possible to do so with some of the older guides available online.[15,16]
Stacking Considerations
First, don't waste such a tool by having a garbage diet:

Even though prohormones are technically 'stronger', there's absolutely no reason to ditch your creatine, betaine, glutamine+l-alanine, and LCLT. Click the link to see our next-generation muscle-building supplements that discuss these all-in-one products and talk about other new natural compounds to run during your PCT to keep your gains!
Bring your protein, quality carbs, and healthy fats to the table bigtime. It would be ludicrous to run this on a low protein diet - so find a way to get like 250g protein per day, every day. Of course, our favorite protein powders can help there, but you're going to want to have your bulk cooking A-game on. Bake yourself a ton of chicken breasts, hit the crock pot, have the SnapWare / Tupperware ready, etc...
As for stacking, this is no replacement for the standard muscle builders like creatine and betaine. No reason not to take them. On top of that, L-Carnitine L-Tartrate is excellent, as it is supportive of androgen receptors in muscle cells.[17,18]
All-in-One Muscle Building Supplements
The easiest way to get these in are the new "all in one" daily muscle building supplements, which we discuss in the preamble of our next generation muscle building supplements article. In that article, there are also other ingredients explored that you can stack in, but they will definitely run your budget up and won't be as effective as the 1-DHEA here anyway.
We always recommend one new thing at a time, so keep the experimentation light, given that Hi-Tech Pharma's 1-AD isn't cheap in the first place.
MOST IMPORTANT... on cycle support in your stack
However, the most important natural supplement to have is the on-cycle-support mentioned multiple times in this document. There are several key ingredients to look for in order to help support the liver, kidneys, healthy estrogen levels, prolactin levels, and more.
Without a doubt, this should be taken alongside 1-DHEA, yet that might not even be enough -- which brings us to our next section:
Is Post Cycle Therapy (PCT) Required?
YES. As with all anabolic steroids, a proper post cycle therapy with either Nolvadex (tamoxifen) or Clomid (clomiphene citrate) is required. There is simply nothing over the counter that is going to be able to bring your testosterone levels back to your prior levels without it.[3]
Although there might be some supplements that could help with that goal, you are taking it completely to chance without getting these drugs. We will always take a better be safe than sorry approach, and that's why you should see your endocrinologist and have prescriptions ready.
A proper post cycle therapy would involve an example protocol of running 4 weeks of Nolvadex starting with 40mg per day for two weeks, followed by another two weeks of 20mg per day. Generally with prohormones, the use of more advanced compounds like hCG (human chorionic gonadotropin) shouldn't be needed.
You can read our guide on post cycle therapy which discusses both the details on the prescription-grade and additional support supplements to consider:
The cortisol component
It's also ideal during the post cycle period to take something that would help keep cortisol levels in check such as phosphatidylserine[19] and a month of whatever natural muscle building supplements mentioned above that you can afford to help preserve your lean mass in a catabolic environment.
One additional supplement not mentioned in the muscle-building article linked there is HMB which would theoretically help preserve lean mass in a catabolic environment, making it an ideal addition to a PCT cycle. HMB has been making a comeback now that it's not ridiculously expensive!
Keep your protein intake high and lower training volume while your testosterone levels come back to their pre-cycle levels.
Get blood tests -- Seriously!!
It is also absolutely critical to get a full panel blood test and urinalysis to make sure your liver, kidneys, and cardiovascular health are all in good working order. Checking your testosterone, estradiol, cortisol, and IGF are also highly recommended. So many lifters who opt to use anabolic steroids ignore this important step and risk long-term detriment to their health, not to mention put a lot of time trying to guess whether or not their testosterone levels are back up and their estrogen isn't sky high. Have appointments ready with the endocrinologist too. Flying blind is never worth risking!
With this information you can make simple determinations as to what you may need to supplement with or modify diet, training or consume other ancillary drugs to get your health back in line. Don't let it be guesswork!
If we've scared you with this section, then good - you're not ready to step into this realm. Come ready or don't come at all.
On cycle therapy: protect your liver, kidneys, and cortisol levels
As mentioned above in the stacking section, a quality cycle support supplement is critical to take while on 1-andro, especially since we know that it can be damaging to the liver, kidney, and fatty acid levels - even if only temporarily. Many of the side effects can be alleviated (or at least combated) with proper use of ancillary drugs or supplements with ingredients such as NAC, milk thistle, saw palmetto, trans-resveratrol, a solid multivitamin, electrolytes (we are deficient in magnesium as it is), etc. This is known as "on-cycle support".
The estrogenic side effect considerations
See your doctor / endocrinologist and get blood tests! You can't eyeball this and it's not worth messing up! Image courtesy Wikimedia
While estrogen wouldn't be a major problem with a compound such as 1-testosterone, the fact that 1-DHEA is converting through multiple pathways might result in one of its substrates converting to estrogen prior to conversion through one of the enzyme pathways it takes. Whether or not this actually happens is not entirely known, so it's best to be safe than sorry and have a legit prescription-strength PCT on the ready.
In that same vein, expensive aromatase inhibitors would be unnecessary here, as an estrogen receptor antagonist like Nolvadex would do the trick. Propecia (finasteride) is also commonly kept on hand to prevent prostate enlargement along with hair loss. However, again, this should not be as much of an issue with 1-testosterone since finasteride inhibits testosterone from making the conversion to DHT - 1-testosterone does not make this conversion and makes this compound virtually unnecessary.
What about Prolactin-based side effects?
Finally, the last thing to beware of is increased prolactin levels - some steroids are recognized in the body as progestins, and they may cause an increase in prolactin.[20] This is the protein that enables females to produce breast milk… something we clearly don't want to deal with (it's known as "testosterone-induced hyperprolactinemia" when it occurs in males).
When this occurs, it can create a negative feedback loop since the prolactin might also suppress testosterone production.
Unfortunately, we're not yet sure how 1-Andro -> 1-Testosterone affects prolactin levels.
Plus, there are plenty of people who claim that prolactin-induced gyno is a myth.[21,22]
It all may depend on the amount of estrogen created. But if 1-Andro does affect prolactin levels, one solution is to boost your dopamine production, which is a prolactin inhibitor. To do this, taking mucuna pruriens along with Vitamin B6 (pyridoxine) could help.[23,24,25] This is discussed in detail in the prolactin section of our post cycle therapy blog post. Both of these might also combat the anecdotal lethargy that many users used to claim to get with 1-Testosterone, so they're not a bad idea anyway.
So, while many believe that prolactin concerns are overstated in discussion forums by various AAS users and may not be that big of a deal - is some mucuna and B6 in your stack really going to hurt?
Once again... before/during/after blood tests are where you will spot this kind of thing.
Lethargy or loss of libido?
Earlier in this document, we discussed how former 1-testosterone users occasionally experienced lethargy and loss of libido. Since 1-DHEA seems to improve mood, we're not sure this is a major concern anymore, but if it happens, there are additional components you can consider.
For instance, caffeine and other stimulants can with the lethargy, and possibility some over the counter herbal remedies for libido can be considered (including horny goat weed, maca, and D-aspartic acid), but that's up to the individual user's discretion as to what they feel is necessary.
Point is, if it does happen, there may not be a hormonal "fix all" solution to the tiredness and lack of libido that 1-Andro imparts. A user would be welcome to experiment with 4-Andro to see if it can possibility balance out the negatives, or just suffer through them and wait until they subside once the cycle is over.
Conclusion

Most prohormones don't have legit studies on them, let's consider ourselves lucky with this one.
So here we are. The next round of "legal prohormones" are out, and they're finally out from companies whose manufacturing practices we actually trust and take it to the next level.
Hopefully we've made it clear that this is an advanced compound. 1-Testosterone is the big daddy that novices might not be ready for. Again, if you're new to the world of prohormones and have never used one before, this is honestly not where we'd start - we'd actually prefer 1,4DHEA - an article we hope to have in the future.
In the quest to work around the FDA's and DEA's latest laws, there are new ramifications to consider. We feel that we've been completely fair and honest in this post - it's not all flowers and puppies when you enter this world. You need to know what you're doing, get help, get blood tests, talk to a doctor, and work + eat your ass off.
Once again, if this post scared you, then you're not ready. If you're not 23 or older, stick with the natural stuff, such as in our muscle building supplements article. But if you're ready to go, we hope we've helped you out, and saved you a bit of trouble and worry.
There's no doubt, 1-Androsterone is going to open you to the possibility of gains that you've never experienced naturally. Nobody is arguing that. But whether or not the risk/reward is worth it to you is a personal decision. Just do it right, or please don't do it at all.
This article was co-written by Pogue.
References
- United States Department of Justice; Drug Enforcement Administration; "Rules - 2005 - Implementation Of The Anabolic Steroid Control Act Of 2004"; December 16, 2005; Retrieved from https://www.deadiversion.usdoj.gov/fed_regs/rules/2005/fr1216.htm
- Granados, J. et al; "Prohormone Supplement 3 -Hydroxy-5 -Androst-1-En-17-One Enhances Resistance Training Gains But Impairs User Health"; Journal of Applied Physiology; 116.5 (2013): 560-569; Retrieved from https://jap.physiology.org/content/jap/116/5/560.full.pdf (via https://jap.physiology.org/content/116/5/560.long)
- Llewellyn, W; ANABOLICS, 10th Edition; Molecular Nutrition; Amazon Kindle E-Book; August 4, 2011; Retrieved from https://blog.priceplow.com/out/anabolics-william-lewellyn-10th-edition
- Parr, Maria K. et al; "Seized Designer Supplement Named '1-Androsterone': Identification As 3Β-Hydroxy-5Α-Androst-1-En-17-One And Its Urinary Elimination;. Steroids; 76.6 (2011): 540-547; Retrieved from https://www.researchgate.net/profil
![1-Andro Results: Those are some serious lean mass gains. These are true steroid-like gains!<sup>[2]</sup>](https://blog.priceplow.com/wp-content/uploads/1-andro-results.png)
![1-DHEA's effects on anger.<sup>[2]</sup> Can someone under 23 handle it? We suggest not finding out](https://blog.priceplow.com/wp-content/uploads/1-andro-anger-300x355.png)